CryoLetters Volume 46 - Issue 2
CryoLetters 46 (2), 67-73 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110112
PERSPECTIVE: Strategies used by organisms to survive very cold climates – student’s guide
S. P. Chesterfield1, H. V. Joyce1, T. D. Fooks1 and P. W. Wilson1,2,3*
- One School Global, Sydney, Australia
- Scripps Institution of Oceanography, University of California San Diego, USA
- Department of Physics, The University at Albany, NY, USA
*Corresponding author’s E-mail: peterwwilson@yahoo.com
Abstract
We briefly examine how cold-hardiness in general, including freeze-tolerance, freeze-avoidance and dehydration strategies allow survival in cold climates, through the eyes of some specific insects and fish. Strategies do vary with geography and latitude, even between two types of insects living in the same area. We look at ice nucleation proteins to enhance freezing and antifreeze proteins to help avoid ice formation or, in some cases, to hinder what is known as recrystallization, as a frozen organism thaws.
Keywords: cold-hardiness; freeze-tolerance; antifreeze; nucleation; supercooling

CryoLetters 46 (2), 74-81 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110312
PERSPECTIVE: Challenges in bird cryopreservation
Juan Pérez-Rivero
- Departamento de Producción Agrícola y Animal, Universidad Autónoma Metropolitana Unidad Xochimilco Calzada del Hueso 1100 Coyoacan, 04960 Ciudad de México, Mexico
*Corresponding author’s E-mail: jjperez1_1999@yahoo.com and jperezr@correo.xoc.uam.mx
Abstract
Cryopreservation is a fundamental technique for preserving the structural and functional integrity of biological material, particularly for the conservation of genetic resources in avian species. Since its development in the 1940s, this technology has advanced significantly, although challenges persist, primarily due to the unique morphology of avian sperm, which complicates cryoprotectant penetration and increases the risk of structural damage. Overcoming these challenges is crucial for improving semen preservation, supporting the sustainability of avian species, and contributing to conservation efforts. In domestic production birds, cryopreservation is essential for maintaining genetic diversity. However, these species often exhibit low tolerance to the freezing process, primarily due to the high concentration of polyunsaturated fatty acids in their sperm membranes, making them susceptible to oxidative damage. This has driven research aimed at developing more effective cryoprotectants and techniques to enhance semen quality post-thaw. Wild birds, particularly endangered species, face additional challenges in cryopreservation. These species are often managed in captivity to prevent extinction, with artificial insemination serving as a valuable tool. However, artificial insemination is constrained by low post-thaw motility rates, even when advanced cryoprotectants are employed. Research indicates that certain cryopreservation media can improve sperm motility and fertility rates, although further optimization of these methods is required. The future of avian semen cryopreservation will concentrate on customizing extenders and cryoprotectants, optimizing freezing techniques, and improving post-thaw semen quality. These advancements are essential for enhancing commercial poultry production and for the conservation of endangered species. Research in this area is expected to evolve over the next decade, developing effective solutions to address both commercial and conservation needs.
Keywords: domestic birds; wild birds; raptors; cryoprotectants; semen extenders

CryoLetters 46 (2), 82-91 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110512
A ten-year retrospective on the efficacy of droplet vitrification for cryobanking of Allium ramosum L. germplasm
Subhash Chander1#, Ravi Gowthami1#, Ruchira Pandey1#, DA Deepak2 and Anuradha Agrawal1,3*
- Tissue Culture & Cryopreservation Unit, ICAR-National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India
- Division of Plant Genetic Resources, ICAR- Indian Agricultural Research Institute, Pusa Campus, New Delhi-110012, India
- Present Affiliation: National Agricultural Higher Education Project (NAHEP), Krishi Anusandhan Bhawan-II, Indian Council of Agricultural Research (ICAR), Pusa Campus, New Delhi-110012, India
*Corresponding author’s E-mail: Anuradha.Agrawal@icar.gov.in
#Authors contributed equally to this work and share first authorship
Abstract
Background
Allium ramosum is an important member of the genus Allium, which is commonly known as Chinese chive or fragrant-flowered garlic. Conserving the genetic diversity of different species of Allium is crucial, and cryopreservation has emerged as an important strategy for long-term conservation of alliums.
Objective
To develop a reliable protocol for the cryoconservation of A. ramosum shoot bases.
Materials and methods
Different parameters, viz. (a) cold-hardening (5°C for 16/8 h photoperiod), (b) PVS2 dehydration (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 and 60 min), (c) pregrowth medium (MM3: MS + 0.1 mg/L NAA + 0.5 mg/L 2iP + 10 mg/L spermidine + 3% sucrose; MM10: MS + 0.1 mg/L NAA + 0.5 mg/l 2iP + 10 mg/L spermidine + 10% sucrose) and (d) preculture duration (1, 2, 3, 4 and 5 days) were tested using a vitrification technique.
Results
Shoot bases excised from 4-wk old in vitro cultures that had been cold-hardened at 5°C (16/8 h photoperiod) and precultured on MM10 with 10% sucrose at 5°C for 3 days resulted in highest post-thaw regrowth of 43% after conventional vitrification. However, when droplet-vitrification was used, post-thaw regrowth was increased to 77%. Retesting of shoot bases after 10 years of cryobanking, revealed no significant difference in the post-thaw regrowth of A. ramosum.
Conclusion
This is the first report of the long-term cryopreservation of A. ramosum shoot bases using vitrification and droplet-vitrification techniques.
Keywords: Allium ramosum; cryopreservation; droplet vitrification; preculture; vitrification.

CryoLetters 46 (2), 92-97 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110712
Formation of an outer glassy shell hinders the drying of trehalose droplets
Gen Pei, Yu Zhang, Pei Zhou and Cai Gao*
- Department of Refrigeration and Cryogenics Engineering, Hefei University of Technology, Hefei, China
*Corresponding author’s E-mail: gaocai@hfut.edu.cn
Abstract
Background
Airflow drying of mammalian cells has not been successful yet, with one obstacle being the improper drying time of cells-laden trehalose droplets.
Objective
To evaluated the major factors affecting the drying kinetics of aqueous trehalose droplets.
Materials and methods
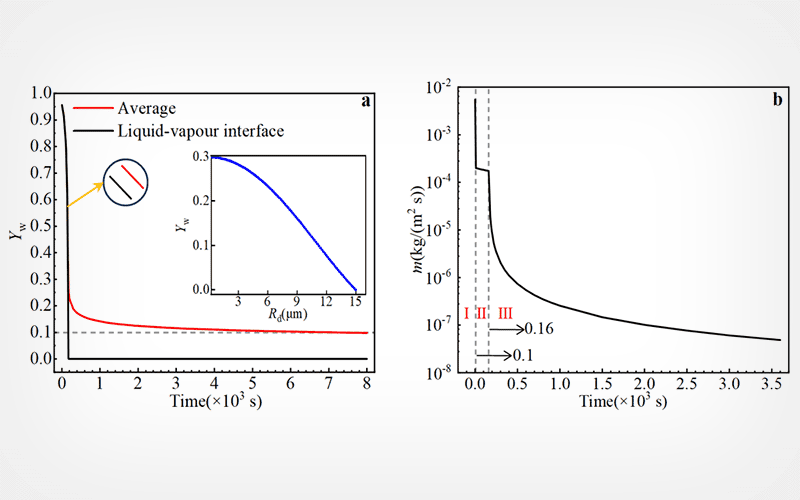
A numerical analysis was applied to the evaporation behavior of aqueous trehalose droplets for the preservation of biomaterials. Factors such as convection caused by droplet contraction, vapor diffusion, and Stefan flow around droplets were taken into account in the analysis.
Results
Reducing the size and initial concentration of the droplets helps to achieve rapid drying of droplets. Forced convection can effectively enhance the initial drying rate of droplets, which may mitigate cell damage caused by hypertonic solutions. Upon drying, however, the rapid formation of an outer glassy shell inhibits the further drying of trehalose droplets.
Conclusion
Simple enhancement of convection does not facilitate complete droplet vitrification due to the formation of an outer glassy shell that prevents water movement.
Keywords: aqueous trehalose droplet; drying kinetics; glass transition; numerical model
CryoLetters 46 (2), 98-107 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110912
Effect of Hericium erinaceus polysaccharides on the cryopreservation of bull semen
M.I. Sergushkina1*, T. V. Polezhaeva1, , O. N. Solomina1, A.N. Khudyakov1, O. O. Zaitseva1 and Y.I. Nazarova2
- Institute of Physiology of Komi Science Centre of the Ural Branch of the Russian Academy of Sciences, FRC Komi SC UB RAS, Syktyvkar, 167982, Russia
- Federal Agricultural Research Center of the North-East named N.V. Rudnitsky, Kirov, 610007, Russia
*Corresponding author’s E-mail: mara.kovalkova@mail.ru
Abstract
Background
The field of sperm cryopreservation requires the search for and development of new effective cryopreservatives with low toxicity.
Objective
The purpose of this study was to evaluate the characteristics of bull spermatozoa subjected to freezing and storage at -80 °C for 28 days in a diluent with the addition of Hericium erinaceus polysaccharides.
Materials and methods
The sperm was frozen under the protection of a complex cryoprotectant and stored in an electric freezer at -80 °C for 28 days. Before cooling and after defrosting, the biological parameters of the sperm were analyzed using the Argus-CASA system.
Results
It was established that two fractions isolated from H. erinaceus (PF1, PF2) are heteropolysaccharide peptides with a high content of glucose, xylose, mannose, galactose and arabinose. It was shown that before cooling, when a fraction obtained from dry fruiting bodies of H. erinaceus (PF2) was added to the sperm, the number of progressively motile sperm with intact DNA decreased, but their kinematic parameters did not change. After thawing, after 28 days of storage at -80 °C, an increase in the number of progressively motile sperm with intact DNA and their kinematic parameters was observed.
Conclusion
Thus, fungal polysaccharides are obviously underestimated macromolecular substances with enormous cryostabilization potential, and polysaccharide fractions from dried fruiting bodies of H. erinaceus may in the future find wide application in the composition of new cryopreservation media.
Keywords: cooling; Hericium erinaceus; polysaccharides; spermatozoa

CryoLetters 46 (2), 108-115 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110212
Verification of the decrease in cell recovery after freezing and thawing due to suboptimal shipping using nine cancer cell lines and the differences in impacts between the cell lines
Masanori Sato1#* , Yayoi Nakata1#, Mariko Noguchi1#, Satohiko Araki2 and Yasunori Matsuo1
- NITE International Patent Organism Depositary (IPOD) 2-5-8, Kazusakamatari, Kisarazu-shi, Chiba, 292-0818, Japan
- University of Nagoya, Furo-cho, Chikusa-ku, Nagoya, 464-8601, Japan
*Corresponding author’s E-mail: sato-masanori2@nite.go.jp
#The three authors contributed equally to this work
Abstract
Background
The impacts of suboptimal shipping conditions during transport on cell viability, recovery, and function of cryopreserved samples, have not been well studied.
Objective
The impacts of suboptimal shipping on viability and recovery after the freezing and thawing were investigated using nine cancer cell lines, with particular reference to the approximate level of exposure temperature and exposure time at which adverse effects occur, and whether there are differences in sensitivity between cell types.
Materials and methods
The adverse effects of any set of suboptimal shipping conditions (-80°C for 7 d, -65°C or -50°C for 1, 3, and 7 d) on nine cancer cell lines (CHO-K1, COS-1, HeLa, HepG2, HL-60, Jurkat, MCF7, MDCK, 293T) were compared with data obtained during storage in liquid nitrogen.
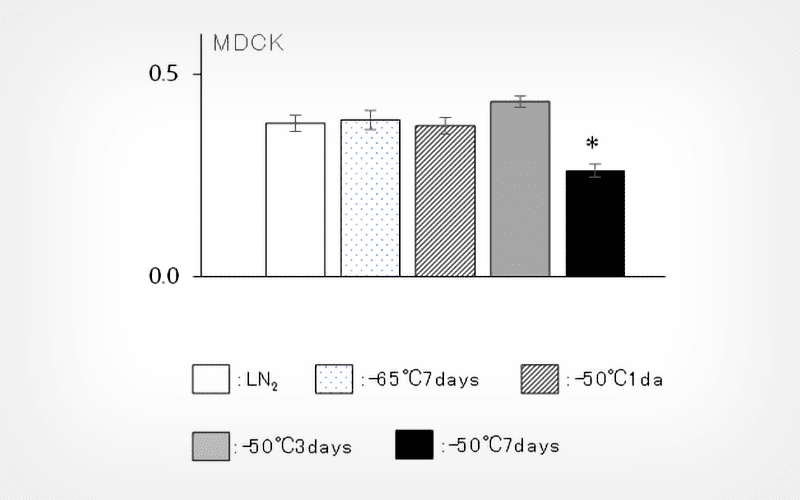
Results
No statistically significant decrease in viability was observed in seven of the nine cell lines after freezing and thawing. On the other hand, a statistically significant decrease in the cell recovery was observed after 2 d post freezing and thawing in the nine cell lines, except CHO-K1 at higher exposure temperatures and longer exposure times. Visualization of the adverse effects on the cell lines using a heat map showed that the impacts tended to be more pronounced under the condition of exposure at -50°C for three or more days.
Conclusion
These results will contribute to the development of standardized protocols and best practices for the optimal shipping of frozen animal cells.
Keywords: cancer cell line; cryopreservation; optimal sample shipping; temperature change
CryoLetters 46 (2), 116-125 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110412
Ultrastructure assessment of oocyte from mouse with polycystic ovary syndrome following cryopreservation
Sajedeh Afzalnia, Fatemeh Ghasemian*, Haniyeh Saadat Maryan and Tooba Mirzapour
- Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran
*Corresponding author’s E-mail: ghasemian@guilan.ac.ir
Abstract
Background
The polycystic ovary syndrome (PCOS) is a substantial obstacle to female fertility due to ovulation inhibition. Oocyte cryopreservation is crucial for preserving fertility in women with fertility-compromising disorders such as PCOS.
Objective
In this study, the ultrastructural damages of oocytes were evaluated following freezing from PCOS mouse model.
Materials and methods
This experimental study was conducted on 30 adult NMRI mouse. The study comprised three groups: 1) unfrozen PCOS oocytes; 2) vitrified-thawed control oocytes; and 3) vitrified-thawed PCOS oocytes. Transmission electron microscopy was employed for ultrastructure examination across all groups. Moreover, the expression of apoptotic genes, including BAX and Bcl2, was assessed using real time-quantitative polymerase chain reaction (RT-PCR).
Results
The oocyte cryopreservation process had a high impact on the destruction of ooplasm cortical granules, Golgi complexes and mitochondria in vitrified-thawed PCOS oocytes compared to the other groups. In PCOS oocytes, particularly those that were vitrified-thawed, there was a notable increase in vacuolation, with a higher abundance of larger and more numerous vacuoles observed compared to the control group. The vitrified-thawed PCOS group also exhibited a notable increase in the expression of the apoptotic gene compared to the other groups (p<0.05).
Conclusion
A precise evaluation of oocyte cryopreservation is imperative for improving this technique and for producing high-quality oocytes with enhanced fertility potential. This study contributes valuable insights into understanding the intricate relationship between PCOS, cryopreservation and oocyte quality.
Keywords: mouse; oocyte; PCOS; TEM; vitrification

CryoLetters 46 (2), 126-134 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110612
Fortification of semen extender with mifepristone improves the cryo-survival of cattle spermatozoa
Arun KUMAR, Vikas SACHAN*, Mukul ANAND, Anuj KUMAR, Neha CHAUDHARY, Garima SINGH, Mohit KUMAR, Jitendra Kumar AGRAWAL and Atul SAXENA
- College of Veterinary Science and Animal Husbandry, UP Pandit Deen Dayal Upadhaya Pashuchikitsa Vigyan Vishwavidyalaya Evam Go-Anusandhan Sansthan, Mathura, UP-281001, India
*Corresponding authors’ E-mail: vikas.vet23@gmail.com
Abstract
Background
Progesterone, which is present in the semen extender as a component of egg yolk is a potential inducer of capacitation in spermatozoa during cryopreservation. An anti-progesterone component in the extender may protect the spermatozoa from being capacitated and pre-acrosome reacted during cryopreservation. It may lead to better quality of post-thaw sperm population for improved conception.
Objective
To investigate the effect of mifepristone on the cryo-survivability of cattle spermatozoa.
Materials and methods
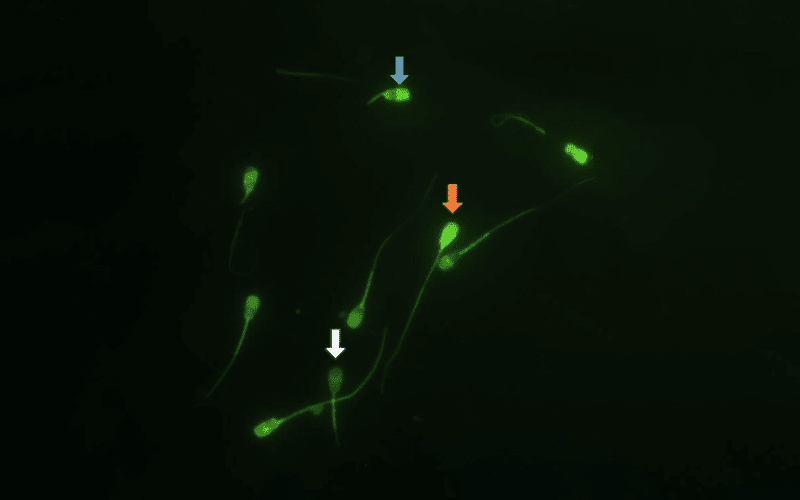
Thirty two semen ejaculates were collected from four Sahiwal bulls and divided into three fractions. These fractions were extended with egg yolk-based TRIS extender supplemented with different concentrations of mifepristone (0, 10 and 20 μM) and subjected to cryopreservation. Cryopreserved semen samples were thawed and evaluated for spermatozoa motion parameters (CASA), viability (flow cytometer), hypo-osmotic swelling test (HOST) responsiveness, capacitation status (CTC), acrosome reaction (flow cytometer) and intracellular calcium ion concentrations (flow cytometer).
Results
There was no definitive effect of mifepristone on sperm motility and kinematics. However, the semen samples which were treated with mifepristone showed significantly higher spermatozoa viability and HOST responsiveness. Mifepristone also protected spermatozoa from being cryo-capacitated during the preservation process. Higher percentages of uncapacitated and acrosome intact spermatozoa were found at the post-thaw stage in comparison to the untreated group. Mifepristone-treated groups showed fewer spermatozoa with high intracellular calcium levels.
Conclusion
A 10 μM concentration of mifepristone has better potential to protect the spermatozoa from progesterone-induced cryo-capacitation and premature acrosome reaction during cryopreservation.
Keywords: cryo-survival; cryo-capacitation; mifepristone; premature acrosome reaction; spermatozoa
CryoLetters 46 (2), 135-142 (2025)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr25210110812
Exposure of carrot seeds to cryopreservation increases root weight and decreases levels of cell wall-linked phenolics
Ysmel Entensa1, Gustavo Lorente1, Lisbet Pérez-Bonachea1, Julio Rodolfo Ynchausti1, Julia Martínez1, Byron E. Zevallos–Bravo2, Barbarita Companioni3, Elliosha Hajari4, Yanier Acosta1, Hugh W. Pritchard5 and José Carlos Lorenzo1*
- Laboratory for Plant Breeding and Conservation of Genetic Resources, Bioplant Center, University of Ciego de Avila, Ciego de Ávila, 69450, Cuba
- Universidad Estatal del Sur de Manabí (UNESUM), Ecuador
- Universidad Autónoma Agraria Antonio Narro, Coahuila, México
- Plant Improvement; Agricultural Research Council-Tropical and Subtropical Crops; Private Bag X11208, Nelspruit, 1200, South Africa
- Chinese Academy of Sciences, Kunming Institute of Botany, Kunming, Yunnan, China (ORCID: 0000-0002-2487-6475)
*Corresponding author’s E-mail: lorenzojosecarlos68@gmail.com
Abstract
Background
The long-term preservation of plant germplasm is critical to ensure a pool of genetic diversity for future breeding efforts and for conservation management.Although it is important to assess whether the biochemical properties and vigour of the germplasm remain unchanged.
Objective
To investigate the potential effects of cryopreservation in liquid nitrogen (LN) as a strategy for conservation of carrot.
Results
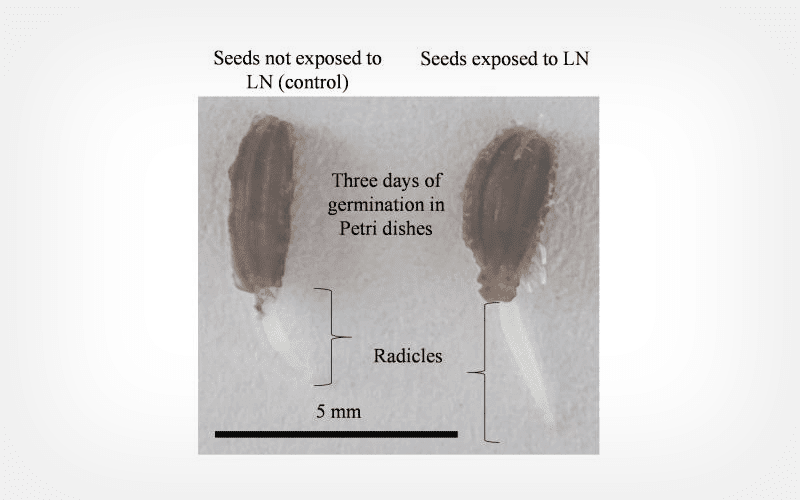
Seeds were exposed to cryogenic temperatures and growth and development was monitored in Petri dishes (for up to 14 days) and in a pot trial (90 days). By day 7, 53% control seeds had germinated compared with 70% seeds exposed to LN. Biochemical attributes were also assessed. Initial differences were observed in the growth rate of plants, where plants originating from seeds exposed to LN emerged faster than those originating from control seeds. However, at the end of the pot trial, differences were only observed in belowground biomass (i.e., mass of carrots). Biochemical evaluations showed that carotenoids and cell wall-linked phenolics were elevated in carrots produced from seeds exposed to LN.
Conclusion
Overall, the results show that cryopreservation can be used as a viable strategy for long-term preservation of carrot germplasm.
Keywords: abiotic stress; carrot quality; climate change; cryopreservation; vegetables