CryoLetters Volume 45 - Issue 2
CryoLetters 45 (3), 139-148 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24310110112
PERSPECTIVE: The cold chain delivery of organs for transplantation: from research laboratories and individual enthusiasts to pan-global networks in 50 years
Barry J. Fuller & Colin C. Green
- Department of Surgical Biotechnology, Division of Surgery & Interventional Science, University College London, Royal Free Hospital Campus, London NW3 2QG
*Corresponding author’s E-mail: b.fuller@ucl.ac.uk
Abstract
It is some 50 years since the first published reports appeared of ex vivo preservation of organs for transplantation. Over the intervening decades, organ preservation strategies have become one essential component of world-wide clinical transplant services. In the formative years, translational research in organ hypothermic preservation was grappling with the questions about whether static or dynamic storage was preferable, and the practical implications of those choices. Those studies were also informing the newly expanding clinical transplant services. During the middle years, both preservation modalities were practiced by individual group choices. By the 2000’s, the shift in donor demographics demanded a re-evaluation of organ preservation strategies, and now a new era of research and development is promoting adoption of new technologies. In this review we outline many important academic studies which have contributed to this successful history, and give profile to the increasing innovative approaches which are being evaluated for the future.
Keywords: hypothermic machine perfusion; isochoric preservation; nanotechnology; organ preservation; organ cryopreservation; oxidative stress; preservation solutions
CryoLetters 45 (3), 149-157 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24310110312
Cryo-storage of porcine hides at the industrial scale for tissue engineering and regenerative medicine application
Huidan Wang1, Senli Huang2, Yuting Tang2 and Wendell Q. Sun1*
- Institute of Biothermal Science and Technology, School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai, China
- Ruijian Gaoke Biotechnology Corporation, Beijing, China
*Corresponding author’s E-mail: wendell.q.sun@gmail.com
Abstract
Background
The industrial scale cryo-storage of raw tissue materials requires a robust, low-cost and easy-to-operate method that can facilitate the down-stream process.
Objective
The study was aimed to develop the multifunctional protective solutions (MPS) for transportation at ambient conditions and also subsequent cryo-storage below -20°C of raw porcine hides for tissue engineering and regenerative medicine.
Materials and methods
Protective solutions with antimicrobial activity and proteinase-inhibiting activity were developed and tested for its efficacy in preserving the extracellular matrix of porcine dermis from microbial spoilage, proteolytic degradation, freeze damage and excessive dehydration during shipping and cryo-storage. The MPSs contained phosphate-buffered saline with ethylene diamine tetra acetic acid (EDTA) added as chelator and proteinase inhibitor, as well as glycerol or maltodextrin (M180) as cryoprotectants.
Results
MPSs prepared with EDTA and glycerol or M180 had significant antimicrobial activity and proteinase-inhibiting activity during the period of shipping and handling. Glycerol and M180 prevented eutectic salt precipitation and excessive freeze dehydration upon cryo-storage of porcine hides. Without glycerol or M180, hides could be freeze-dehydrated to the low hydration at ~0.4 g/g dw, and formed irreversible plications after freezing. A critical hydration (0.8~0.9 g/g dw) was observed for the extracellular matrix of porcine dermis, and dehydration to a lower level could impose enormous stress and potential damage. The soaking of porcine hides in MPSs decreased water content as glycerol and M180 entered into dermis. Upon equilibration, the glycerol content in the tissue was about 94% of the incubating glycerol solution, but the M180 content in the tissue was only about 50% of the incubating M180 solution, indicating that M180 did not get into the entire aqueous domain within dermis. MPSs reduced ice formation and increased the unfrozen water content of porcine raw hides upon cryo-storage.
Conclusion
MPSs prepared with EDTA and glycerol or M180 have antimicrobial activity and proteinase-inhibiting activity, which can be used for transportation and cryo-storage of raw hides at the industrial scale. Glycerol at 7.5 % w/v and M180 at 20 % w/v were sufficient to prevent freeze damage and excessive freeze dehydration.
Keywords: freeze dehydration; glycerol; maltodextrin; osmotic dehydration; porcine dermis
CryoLetters 45 (3), 158-167 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24310110512
Aquaporin expression in cryopreserved human sperm: exploring the capabilities of natural deep eutectic solvents (NADES)
Fatemeh Jahanseir1, Fatemeh Ghasemian1* and Ziba Zahiri2,3
- Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran
- Reproductive Health Research Center, Department of Obstetrics and Gynecology, Alzahra Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- Mehr Fertility, Research Center, Guilan University of Medical Sciences, Rasht, Iran
*Corresponding author’s E-mail: ghasemian@guilan.ac.ir
Abstract
Background
Aquaporins (AQPs) are essential proteins that facilitate the rapid movement of water and cryoprotective agents (CPAs) during the cryopreservation process, and ensure the cryo-tolerance of sperm cells.
Objective
This study evaluated the preservation of aquaporin levels in human sperm after undergoing freezing using natural deep eutectic solvents (NADES) as CPAs for cryoprotection.
Materials and methods
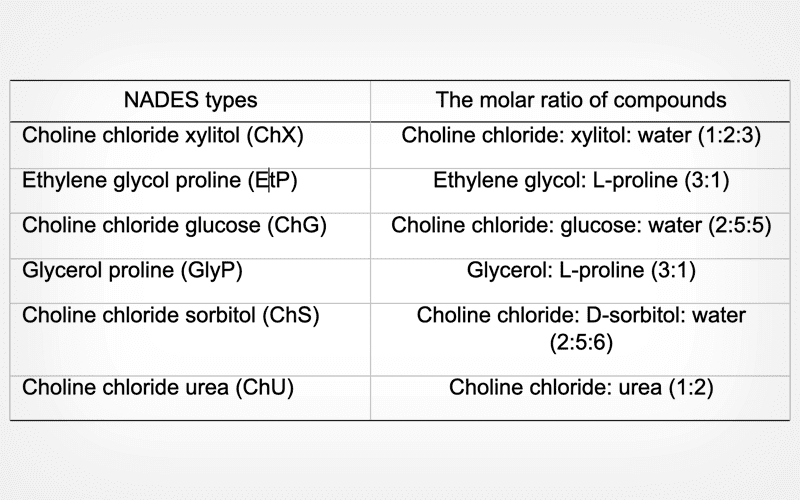
From June 2021 to October 2022, 35 semen samples with normal sperm parameters were acquired from the Mehr Infertility Treatment Institute in Rasht, Iran. The samples were divided into several groups for analysis: control group (not frozen), group frozen with SpermFreeze Solution, and groups frozen with different NADESs, including ChS, ChX, ChU, ChG, GlyP, and EtP. After thawing, various aspects for each group were assessed, including the integrity and condensation of sperm chromatin, viability, motility, integrity of acrosome, and the expression of AQP1, AQP3, AQP7, AQP8, and AQP9 genes.
Results
The analysis of gene expression revealed that freezing with ChS and GlyP preserved the expression of the AQP1 and AQP3 genes compared to the control group. Regarding AQP7 and AQP8, significant differences were not observed in expression levels between certain NADES groups (e.g., ChS, ChU, and GlyP) and the control group. Additionally, samples frozen with specific NADESs, such as ChS, ChG, EtP, and GlyP, exhibited preserved levels of AQP9 expression when compared to the control group.
Conclusion
These findings emphasize the importance of NADES in preserving the expression of aquaporins in cryopreserved human sperm and their important fertility parameters.
Keywords: aquaporins; male infertility; natural deep eutectic solvent; sperm freezing
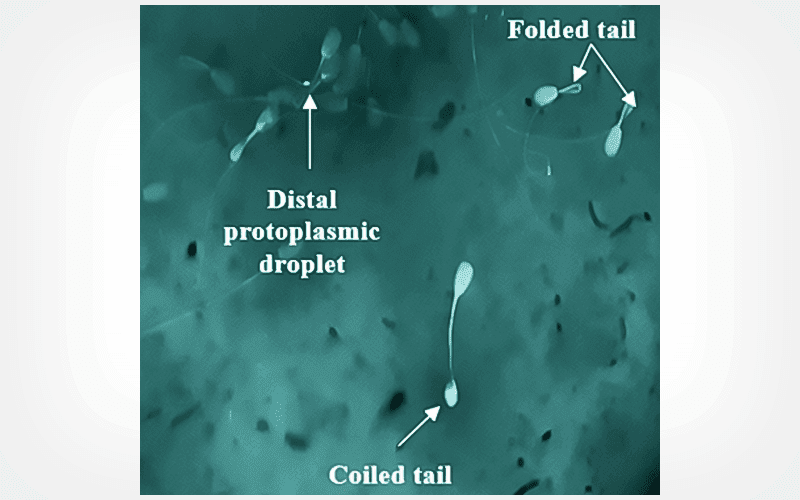
CryoLetters 45 (3), 168-176 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24310110712
Cryopreservation of bovine semen using extract of Cinnamomum zeylanicum
Kabir Alam, Sushant Srivastava, Bhoopendra Singh, Saurabh, Rajesh Kumar*, Rabindra Kumar and Daund Sushant Sakhahari
- Department of Veterinary Gynaecology & Obstetrics, College of Veterinary Science and Animal Husbandry, Acharya Narendra Deva University of Agriculture and Technology Kumarganj, Ayodhya, UP-224229, India
*Corresponding author’s E-mail: drrajesh25@gmail.com
Abstract
Background
Antioxidants minimise oxidative stress and enhance sperm quality in the process of cryopreservation.
Objective
To assess the impact of Cinnamomum zeylanicum extract as an additive during the post-dilution and post-thaw stages of Murrah buffalo semen cryopreservation.
Materials and methods
The semen sample was diluted using Tris-Egg-Yolk-Citric-Acid-Fructose-Glycerol extender and subsequently divided into three groups: Group 1, TEYCAFG without any additives or controls (C); Group 2, TEYCAFG fortified with a 50 μg/mL aqueous extract of cinnamon (T1); and Group 3, TEYCAFG fortified with a 50 μg/mL ethanolic extract of cinnamon (T2). The evaluation included an assessment of progressive motility, live spermatozoa, sperm abnormalities, HOST, CMPT, and enzyme leakage (GOT and GPT) at both the post-dilution and post-thaw stages.
Results
The groups that received cinnamon supplementation demonstrated statistically significant improvements (p<0.05) in various parameters, including an increase in the progressive motility, live spermatozoa, and HOS-positive spermatozoa, as well as greater distance traveled by vanguard spermatozoa compared to the control group. Furthermore, the cinnamon-added groups exhibited a significant decrease (p<0.05) in the percentage of sperm abnormalities and lower enzyme leakage (GOT and GPT) in post-thawed semen.
Conclusion
Aqueous extract of C. zeylanicum at a concentration of 50 μg/mL provides superior protection of sperm structures and functions as compared to both the ethanolic extract of C. zeylanicum at the same concentration and the control group.
Keywords: antioxidant; C. zeylanicum; ethanolic; Murrah buffalo; oxidative stress
CryoLetters 45 (3), 177-184 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24310110212
Liquid nitrogen improves the decellularization effectiveness of whole-ovary
MeyLign Long1#, Zhongying Huang1#, You Yang, Suxiu Sun and Zhun Xiao1*
- Reproductive Medical Department of West China 2nd University Hospital, Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Sichuan University, Chengdu 610041, China
*Corresponding author’s E-mail: xiaozhunok@163.com
#Contributed equally as first authors
Abstract
Background
Ovarian tissue cryopreservation for fertility preservation carries a risk of malignant cell re-seeding. Artificial ovary is a promising method to solve such a problem. However, ovary decellularization protocols are limited. Hence, further studies are necessary to get better ovarian decellularization techniques for the construction of artificial ovary scaffolds.
Objective
To establish an innovative decellularization technique for whole porcine ovaries by integrating liquid nitrogen with chemical agents to reduce the contact time between the scaffolds and chemical reagents.
Materials and methods
Porcine ovaries were randomly assigned to three groups: novel decellularized group, conventional decellularized group and fresh group. The ovaries in the novel decellularized group underwent three cycles of freezing by liquid nitrogen and thawing at temperatures around 37°C before decellularization. The efficiency of the decellularization procedure was assessed through histological staining and DNA content analysis. The maintenance of ovarian decellularized extracellular matrix(ODECM) constituents was determined by analyzing the content of matrix proteins. Additionally, we evaluated the biocompatibility of the decellularized extracellular matrix(dECM) by observing the growth of granulosa cells on the ODECM scaffold in vitro.
Results
Hematoxylin and eosin staining, DAPI staining and DNA quantification techniques collectively confirm the success of the novel decellularization methods in removing cellular and nuclear components from ovarian tissue. Moreover, quantitative assessments of ODECM contents revealed that the novel decellularization technique preserved more collagen and glycosaminoglycan compared to the conventional decellularized group (P<0.05). Additionally, the novel decellularized scaffold exhibited a significantly higher number of granulosa cells than the conventional scaffold during in vitro co-culture (P<0.05).
Conclusion
The novel decellularized method demonstrated high efficacy in eliminating DNA and cellular structures while effectively preserving the extracellular matrix. As a result, the novel decellularized method holds significant promise as a viable technique for ovarian decellularization in forthcoming studies.
Keywords: chemical reagent; decellularized extracellular matrix; liquid nitrogen; whole ovary
CryoLetters 45 (3), 185-193 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24310110412
Visualization of intracellular ice formation and growth in mouse oocytes
Xin Li1,2,3, Shuyong Zhang1,2,3, Yuqi Zhang1,2,3 and Xinli Zhou1,2,3*
- Institute of Biothermal Science & Technology, University of Shanghai for Science and Technology, Shanghai 200093, China
- Shanghai Co-innovation Center for Energy Therapy of Tumors, Shanghai 200093, China
- Shanghai Technical Service Platform for Cryopreservation of Biological Resources, Shanghai 200093, China
*Corresponding author’s E-mail: zjulily@163.com
Abstract
Background
Characterization of intracellular ice formation (IIF) in oocytes during the freezing and thawing processes will contribute to optimizing their cryopreservation. However, the observation of the ice formation process in oocytes is limited by the spatiotemporal resolution of the cryomicroscope systems.
Objective
To observe the intracellular icing of oocytes during cooling and rewarming, and to study the mechanism of formation and growth of intracellular ice in oocytes.
Materials and methods
Mouse oocytes were frozen at different cooling rates to induce intracellular ice formation using a cryomicroscopy system consisting of a microscope equipped with a cryogenic cold stage, an automatic cooling system, a temperature control system, and a high-speed camera. The growth patterns of intracellular ice in oocytes were analyzed from the images recorded. Finally, the growth rate of intracellular ice formation in oocytes was calculated using an automatic intracellular ice tracking method.
Results
The IIF temperature decreased gradually with the increase in cooling rate. Initiation sites of IIF could be classified into three categories: marginal type, internal type and coexisting type. There was a strong predominance for ice crystal initiation site in the oocytes, with up to 80% of the initiation sites located in the marginal region. The intracellular ice growth modes of darkening and twitching cells were characterized by "spreading" and "clustering", respectively. In addition, twitching cells started to recrystallize during rewarming, while darkening cells did not. The instantaneous maximal growth rate of ice crystals in twitching cells was about 10 times higher than that in darkening cells.
Conclusion
By visualising the growth of ice crystals in mouse oocytes during cooling and rewarming, we obtained valuable information on the kinetics of ice formation and melting in these cells. This information can help us understand how ice formation and melting affect the viability and quality of oocytes after cryopreservation.
Keywords: darkening cells; intracellular ice formation; oocyte; twitching cells; visualization






