CryoLetters Volume 45 - Issue 2
CryoLetters 45 (2), 69-87 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110112
PERSPECTIVE: Targeted development and optimization of small-molecule ice recrystallization inhibitors (IRIs) for the cryopreservation of biological systems
Leah E. McMunn1#, Ellyssa M. Walsh1# and Robert N. Ben1*
- Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, ON, Canada
*Corresponding author’s E-mail: robert.ben@uottawa.ca
#Contributed equally as first authors
Abstract
Despite the routine use of cryopreservation for the storage of biological materials, its outcomes are often sub-optimal (including reduced post-thaw viability, recovery, and functionality) due to the damage caused by uncontrolled ice growth. Traditional cryoprotective agents (CPAs), including dimethyl sulfoxide (DMSO), fail to prevent damage caused by ice growth and concerns over CPA cytotoxicity have fostered an increased interest in developing improved CPAs and cryoprotection strategies. The inhibition of ice recrystallization by natural antifreeze (glyco)proteins [AF(G)Ps] to improve cryopreservation outcomes has been examined; however, the ice binding properties of these substances and their challenging large-scale production make them poor CPA candidates. Therefore, the development and deployment of biocompatible, small-molecule ice recrystallization inhibitors (IRIs) for use as CPAs is a worthwhile objective. Extensive structure-activity relationship studies on AF(G)Ps revealed that simple carbohydrate derivatives could inhibit ice recrystallization. It was later discovered that this activity could be fine-tuned by delicately balancing the molecule’s hydrophobicity and hydrophilicity. Current generation small-molecule IRIs have been meticulously designed to avoid binding to the surface of ice and subsequent biological testing (for both cytotoxicity and cryopreservation efficacy) has demonstrated significant improvements to the cryopreservation outcomes of several cell types. However, an individualized cell-specific approach for the simultaneous assessment of multiple cryopreservation outcomes is necessary to realize the full potential of IRIs as CPAs. This article provides a detailed overview of the development of small-molecule carbohydrate-based IRIs and highlights the crucial cell-specific biological considerations that must be taken into account when assessing cryopreservation outcomes.
Keywords: cryopreservation; cryoprotective agent; ice recrystallization inhibition; small-molecule

CryoLetters 45 (2), 88-99 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110312
RNA-Seq analysis reveals the mechanism in response to cold stress of peach cv ‘Dingjiaba’
Xiaolan Li1,2, Hong Wang1,2* and Xinquan Wu2
- Gansu Academy of Agricultural Sciences, Lanzhou 730070, China
- College of Horticulture, Gansu Agricultural University, Lanzhou 730070, China
*Corresponding author’s E-mail: wrh991130@126.com
Abstract
Background
‘Dingjiaba’ is an important Prunus persica cultivar (cv) mainly grown in the Hexi corridor in northwest China, which has an inherited strong cold tolerance.
Objective
To compare the transcriptome and physiology data of leaves of cvs ‘Dingjiaba’ (D) and ‘Kanoiwa’ (K) following cold treatment at different time periods, in order to gain new insights into the mechanisms of cold adaptation in ‘Dingjiaba’.
Materials and methods
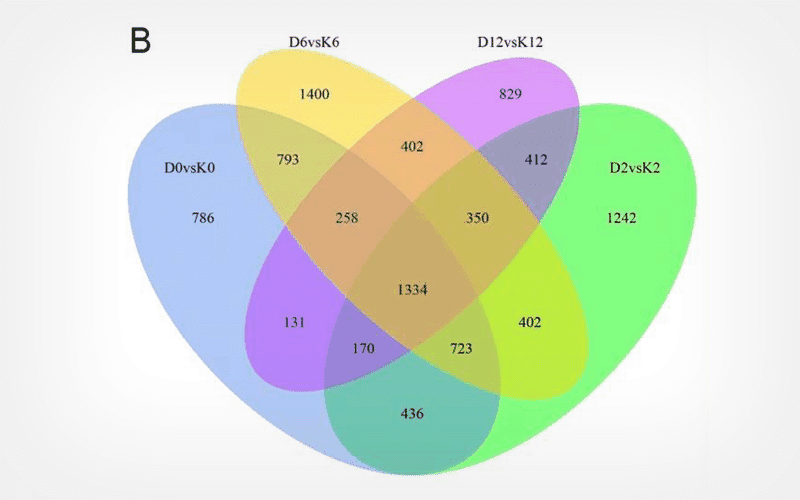
We analyzed the transcriptomic and physiological data of leaves of D and K cvs exposed to 0 h (D0/K0), 2 h (D2/K2), 6 h (D6/K6) and 12 h (D12/K12) cold stress.
Results
Low temperature stress caused membrane damage and led to increased rate of electrolyte leakage and increased MDA content. Cold stress induced the accumulation of soluble sugars, soluble proteins and proline in leaves of both cvs, with a lower increase in K compared to D. Transcriptome analysis identified 4,631, 5,069, 5,662 and 3,886 differentially expressed genes (DEGs) between D0 and K0, D2 and K2, D6 and K6 and D12 and K12, respectively. The differentially expressed genes significantly enriched in metabolic pathways and biosynthesis of secondary metabolites. We further validated the reliability of sequencing data of the RNA-Seq with Real-Time Quantitative PCR, which suggested that the expression trend of the RNA-Seq were same as RT-PCR.
Conclusion
These results provide novel insights into a series of molecular mechanisms underlying physiological metabolism and defense.
Keywords: cold stress; peach; Prunus persica; RNA-Seq
CryoLetters 45 (2), 100-105 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110512
The kinetics of goat sperm is negatively affected after freezing in an extender including zinc oxide nanoparticles
Lúcia Cristina Pereira Arruda1, Gustavo de Oliveira Alves Pinto1*, Gustavo Ferrer Carneiro1 and Maria Madalena Pessoa Guerra1
- Andrology Laboratory (ANDROLAB), Veterinary Medicine Departament, Universidade Federal Rural de Pernambuco, Recife, Pernambuco, Brazil
*Corresponding author’s E-mail: gustavodeoliveiraalvespinto@hotmail.com
Abstract
Background
Nanotechnology can benefit livestock industries, especially through postharvest semen manipulation. Zinc oxide nanoparticles (Np-ZnO) are potentially an example.
Objective
To investigate how the addition of zinc oxide nanoparticles (Np-ZnO) affected the characteristics of post-thawed goat semen.
Materials and methods
Seminal pools from four Saanen bucks were used. Semen was diluted in Tris-egg yolk extender, supplemented with Np-ZnO (0, 50, 100 or 200 μg/mL), frozen and stored in liquid nitrogen (−196°C), and thawed in a water bath (37°C/ 30 s). Semen samples were evaluated for sperm kinetics by computer-assisted sperm analysis (CASA), and assessed for other functional properties by epifluorescence microscopy, such as plasma membrane integrity (PMi), acrosomal membrane integrity (ACi) and mitochondrial membrane potential (MMP).
Results
For total motility (TM), the group treated with 200 μg/mL Np-ZnO was superior to the control. In straight-line velocity (VSL), the control was better than the group containing 200 μg/mL of Np-ZnO. For average path velocity (VAP), the control was higher than with 100 μg/mL Np-ZnO. For linearity (LIN), the control was higher than with 200 μg/mL Np-ZnO. In straightness (STR), the control and 100 μg/mL Np-ZnO were higher than with 200 μg/mL Np-ZnO. In wobble (WOB), the control was better than the 50 μg/mL Np-ZnO treatment. In PMi, ACi and MMP no significant differences were found.
Conclusion
The addition of Np-ZnO (200 μg/mL) to the goat semen freezing extender improved the total motility of cells, whilst negatively affecting sperm kinetics.
Keywords: buck; cryopreservation; nanotechnology; semen
CryoLetters 45 (2), 106-113 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110712
Testing of arctic insect hemolymph as a secondary agent in applied cryopreservation
N. G. Li1
- Department of Cryobiology and Germplasm of Animals from Kazakhstan, Institute of Zoology of Republic of Kazakhstan, 93 al-Farabi Str., Almaty 050060; and Cryoprotect LLC, Innovative Center Skolkovo, Merzlotnaya str., 29-43, Yakutsk 677010, Russia
*Corresponding author’s E-mail: natalia.li@zool.kz
Abstract
Background
Cold hardiness of insects from extremely cold regions is based on a principle of natural cryoprotection, which is associated with physiological mechanisms provided by cryoprotectants.
Objective
Since arctic cold-hardy insects are producers of highly effective cryoprotectants, in this study, the hemolymph of Aporia crataegi L. and Upis ceramboides L. from an extremely cold area (Yakutia) was tested as a secondary component of cryoprotective agents (CPA) for cryopreservation (-80°C) of human peripheral blood lymphocytes and skin fibroblasts.
Materials and methods
Lymphocytes and skin fibroblasts were treated with various combinations of DMSO and hemolymph extract and step-wise cooled to -80°C. Post-cryopreservation cell viability was assessed by vital staining and morphological appearance.
Results
Viability was higher when cells were frozen with a mixture containing DMSO and Upis ceramboides hemolymph compared to the cells frozen in DMSO, while cells frozen with DMSO and Aporia crataegi hemolymph did not survive. The fact that hemolymph of not every cold-resistant insect can be used as a secondary agent along with DMSO indicates that only a unique combination of hemolymph components and its compatibility with cells might result in a positive effect.
Conclusion
Although the use of insect hemolymph as a complementary agent in applied cryopreservation is a problem in terms of practical application, such studies could initiate new trends in the search for the most successful hemolymph-like cryoprotectant systems.
Keywords: cryopreservation; fibroblasts; insect hemolymph; lymphocytes; natural cryoprotectants
CryoLetters 45 (2), 114-121 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110212
A novel vitrified cryopreservation approach with stem cell-laden hydrogel microcapsules
Tao Song and Baolin Liu*
- Institute of Biothermal Technology, University of Shanghai for Science and Technology, Shanghai 200093, China
*Corresponding author’s E-mail: blliuk@163.com
Abstract
Background
Stem cell-laden hydrogel microcapsules construction is important for a wide application in tissue engineering and cell-based medicine, such as building an ideal immune barrier. Challenges are emerging for effectively storing such microcapsules by cryopreservation, and a large proportion of research has been on the cryopreservation of single cells encapsulated into microcapsules without a core-shell structure.
Objective
To achieve the effective cryopreservation of stem cell-laden hydrogel microcapsules with a core-shell structure.
Materials and methods
A novel core-shell alginate hydrogel encapsulation method was used to produce mesenchymal stem cell-laden microcapsules by microfluidic technique.
Results
This microcapsule could inhibit ice formation to achieve vitreous cryopreservation with a low concentration (2 M) of penetrating cryoprotectants.
Conclusion
Cell laden hydrogel microcapsules may have the potential to be the basis of a new strategy of cell cryopreservation and applications.
Keywords: hydrogel; stem cell; vitrification
CryoLetters 45 (2), 122-133 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110412
Cryopreservation and assessment of genetic fidelity Acorus calamus Linn., an endangered medicinal plant
Kiran Parasher1, Charu Sharma1, Shailika Sharma1,2, Shrishti1 and Papiya Mukherjee1*
- Department of Botany, Panjab University, Chandigarh, India, 160014
- Department of Biosciences, University Institute of Biotechnology, Chandigarh University, Mohali, India, 140413
*Corresponding author’s E-mail: pmukherjee.pu@gmail.com
Abstract
Background
Acorus calamus Linn. is a medicinally valuable monocot plant belonging to the family Acoraceae. Over-exploitation and unscientific approach towards harvesting to fulfil an ever-increasing demand have placed it in the endangered list of species.
Objective
To develop vitrification-based cryopreservation protocols for A. calamus shoot tips, using conventional vitrification and V cryo-plate.
Materials and methods
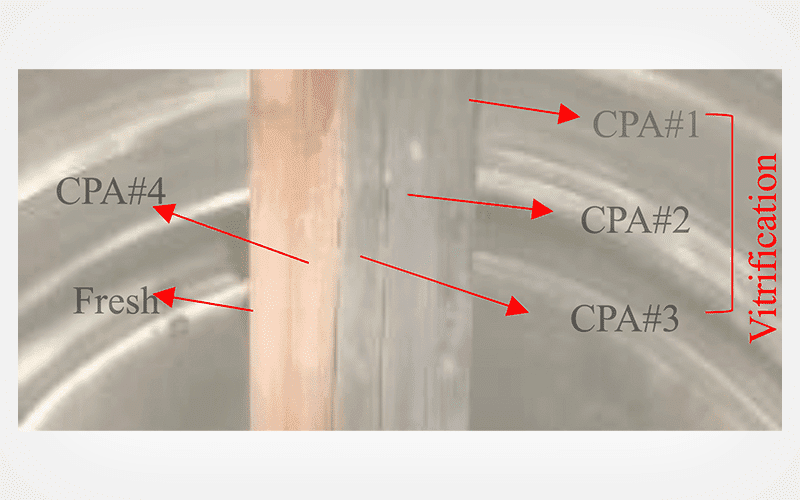
Shoot tips (2 mm in size) were cryopreserved with the above techniques by optimizing various parameters such as preculture duration, sucrose concentration in the preculture medium, and PVS2 dehydration time. Regenerated plantlets obtained post-cryopreservation were evaluated by random amplified polymorphic DNA (RAPD) to test their genetic fidelity.
Results
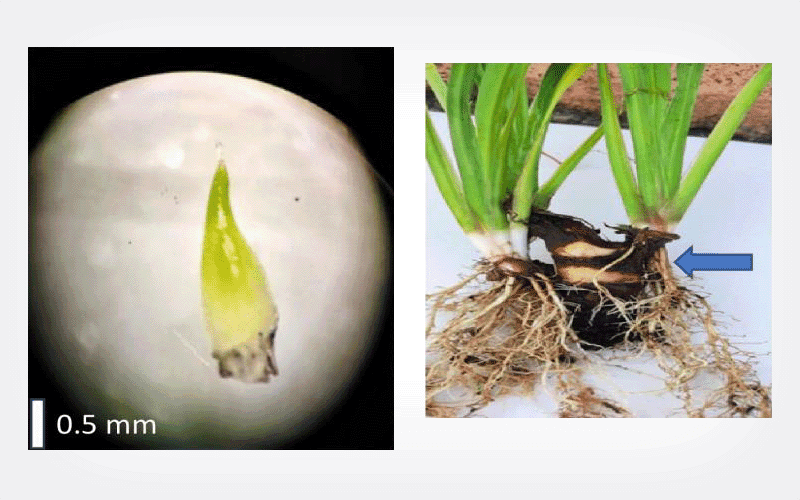
The highest regrowth of 88.3% after PVS2 exposure of 60 min was achieved with V cryo-plate as compared to 75% after 90 min of PVS2 exposure using conventional vitrification. After cryopreservation, shoot tips developed into complete plantlets in 28 days on regrowth medium (0.5 mg L-1 BAP, 0.3 mg L-1 GA3, and 0.3 mg L-1 ascorbic acid). RAPD analysis revealed 100% monomorphism in all cryo-storage derived regenerants and in vitro donor (120-days-old) plants.
Conclusion
Shoot tips of A. calamus that were cryopreserved had 88.3% regrowth using V cryo-plate technique and the regerants retained genetic fidelity.
Keywords: Acorus calamus; genetic fidelity; shoot tips; vitrification; V cryo-plate
CryoLetters 45 (2), 134-138 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24210110612
Optimizing semen cryopreservation in Calomys laucha: a step forward in rodent reproductive research
Carine Dahl Corcini1*, Janice Vilela2, Norton Gatti1, Bruna Mion1, Natália Castro1, Raqueli Teresinha França1 and Antonio Sergio Varela Junior3*
- Center for Research and Education in Animal Reproduction, Federal University of Pelotas, Faculdade de Veterinária, Campus Universitário, S/N - CEP 96160-000
Capão do Leão, RS - Brasil
- Université Catholique de Louvain, Université Catholique de Louvain - Bruxelles, Pôle de Recherche en Gynecologie – Bruxelles, Bélgica
- Institute of Biological Sciences, Federal University of Rio Grande, Campus Carreiros: Av. Itália km 8 Bairro Carreiros- CEP 96203-900 Rio Grande, RS, Brazil
*Corresponding author’s E-mail: corcinicd@gmail.com
Abstract
Background
Examining semen cryopreservation in Calomys laucha offers valuable insights for reproductive research and species conservation.
Objective
To determine the most effective sugar for the cryopreservation of C. laucha semen.
Materials and methods
Using 36 epididymides from C. laucha, semen samples were diluted in a 3% skimmed milk medium supplemented with one of four sugars (glucose, fructose, lactose, or sucrose) at a concentration of 0.3 M. These mixtures underwent a conditioning phase at 37°C for 10 min, cooled to -80°C for another 10 min, and were subsequently stored in liquid nitrogen.
Results
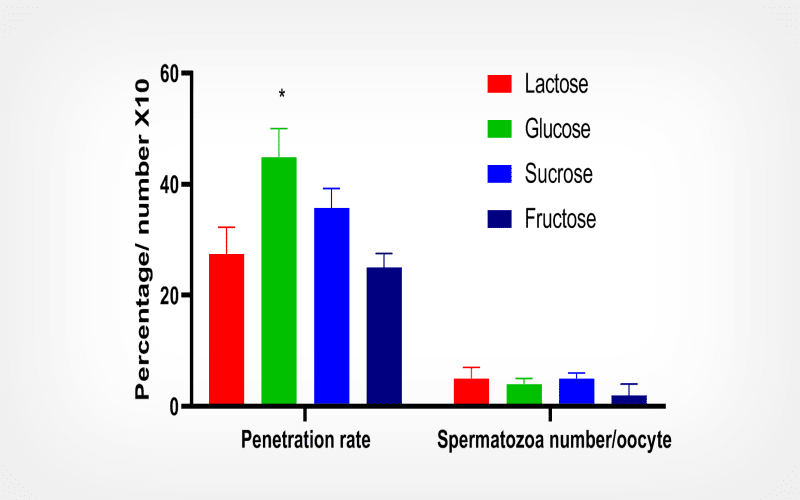
Upon thawing, samples treated with lactose and glucose solutions show superior sperm motility, achieving 8.2% and 10.0% respectively, in contrast to the fructose (2.0%) and sucrose (4.1%) mixtures. Furthermore, samples preserved in glucose registered the highest sperm penetration rates, reaching 44.9%.
Conclusion
Our findings suggest that a cryopreservation medium containing 0.3 M glucose can contribute to the safeguarding C. laucha rodent semen.
Keywords: cryopreservation; cryoprotectants; rodent; sperm quality