CryoLetters Volume 45 - Issue 1
CryoLetters 45 (1), 1–15 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110112
PERSPECTIVE: The comet assay as a method for assessing DNA damage in cryopreserved samples
Beata P. Plitta-Michalak1*, Alice Ramos2,3, Dominika Stępień4, Magdalena Trusiak4 and Marcin Michalak4
- Department of Chemistry, Faculty of Agriculture and Forestry, University of Warmia and Mazury in Olsztyn, Plac Łódzki 4, 10-719 Olsztyn, Poland
- Institute of Biomedical Sciences Abel Salazar (ICBAS), University of Porto (U. Porto), Rua de Jorge Viterbo Ferreira 228, 4050‑313 Porto, Portugal
- Interdisciplinary Center for Marine and Environmental Research (CIIMAR), University of Porto (U. Porto), Avenida General Norton de Matos s/n, 4450-208 Matosinhos, Portugal
- Department of Plant Physiology, Genetics and Biotechnology, Faculty of Biology and Biotechnology, University of Warmia and Mazury in Olsztyn, M Oczapowskiego 1A, 10-721 Olsztyn
*Corresponding author’s E-mail: beata.plitta-michalak@uwm.edu.pl
Abstract
The preservation of the nuclear genome's integrity is paramount for the viability and overall health of cells, tissues, and organisms. DNA, being susceptible to damage under physiological conditions and vulnerable to both endogenous and environmental factors, faces constant threats. To assess DNA damage and repair within individual eukaryotic cells, the comet assay presents itself as a versatile, gel electrophoresis-based, relatively simple, and highly sensitive method. Originally designed to monitor DNA damage and repair within populations of mammalian cells, the comet assay has now found applications across diverse domains, including yeast, protozoa, plants, and invertebrates. This technique has proven invaluable in cryopreservation studies, serving as a valuable adjunct for determining suitable cryopreservation protocols. These protocols encompass choices related to cryoprotectants, sample preparation, as well as storage conditions in terms of time and temperature. In the realm of animal cryopreservation research, the comet assay stands as a gold-standard method for assessing DNA integrity. Nevertheless, when applied in plant-oriented investigations, additional efforts are essential due to the distinct nature of plant cells and associated technical challenges. This review elucidates the fundamental principles underlying the comet assay, discusses its current iterations, and delineates its applications in the cryopreservation of both animal and plant specimens. Moreover, we delve into the primary challenges confronting the comet assay's utility as a monitoring tool in the context of plant sample cryopreservation.
Keywords: comet assay; cryopreservation; DNA stand breaks; 8-oxoG; single-cell gel electrophoresis

CryoLetters 45 (1), 16-27 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110312
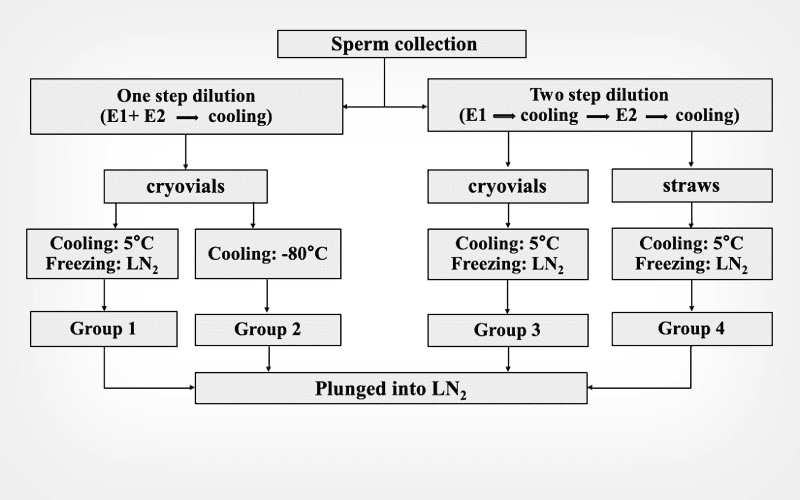
Dog sperm cryopreservation using cryovials and different dilution steps
Saddah Ibrahim1,2, Nabeel Abdelbagi Hamad Talha1,2, Jongki Cho3, Yubyeol Jeon1 and Il-Jeoung Yu1*
- Laboratory of Theriogenology and Reproductive Biotechnology, College of Veterinary Medicine and Bio-safety Research Institute, Jeonbuk National University, Iksan 54596, Republic of Korea
- Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, Sudan University of Science and Technology, Khartoum 11111, Sudan
- College of Veterinary Medicine, Chungnam National University, 34134 Daejeon, Republic of Korea
*Corresponding author’s E-mail: iyu@jbnu.ac.kr
1,2Contributed equally as first authors
Abstract
Background
The conventional sperm freezing method for dog sperm is with straws and includes two-step dilution and a long equilibration time.
Objective
To develop a more efficient freezing method using cryovials.
Materials and methods
Three freezing protocols using cryovials (0.5 mL) were conducted with dog spermatozoa at 1 x 108 sperm/mL: Group 1 spermatozoa were cooled in cryovials and extender 1 (E1) and extender 2 (E1 +1 M glycerol) at 4°C for 50 min and then frozen over LN2 for 20 min; Group 2 sperm was cooled and frozen in cryovials with a mixture of E1 and E2 (1:1) in a deep freezer (-80°C) for 30 min; Group 3 sperm in cryovials and E1 were cooled at 4°C for 20 min, cooled for an additional 20 min after addition of E2 (E1:E2, 1:1), and then frozen using LN2 vapour for 20 min. The control (Group 4) consisted of spermatozoa in straws being frozen using the conventional freezing method using two-step dilution. All groups were plunged and stored in LN2 after freezing and their functional performance and gene expression determined.
Results
Progressive motility and acrosome integrity were highest (P < 0.05) in Groups 2, 3 and 4 (only acrosome integrity). Viability in Group 3 was significantly better that in the other Groups, and the reactive oxygen species (ROS) level and phosphatidylserine (PS) translocation index were significantly lower in Group 2 than the other Groups. The expression of sperm mitochondria-associated cysteine-rich protein (SMCP) and anti-apoptotic B-cell lymphoma 2 (BCL2) genes was highest (P < 0.05) in Group 2 and the expression of pro-apoptotic Bcl2-associated X protein (BAX) was lowest (P < 0.05) in Group 4.
Conclusion
The sperm frozen using cryovials, one step dilution and the deep freezer (Group 2) proved to be a simple and suitable cryopreservation method for dog sperm.
Keywords: cryopreservation; cryovials; dilution steps; dog spermatozoa; freezing method
CryoLetters 45 (1), 28-35 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110512
Effects of stearic acid on the embryo cryopreservation in mouse
TN Igonina1*, TA Rakhmanova1,2,3, AN Omelchenko3, KA Okotrub3, E Yu Brusentsev1, IN Rozhkova1 and S Ya Amstislavsky1
- Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, Lavrentyeva 10, Novosibirsk
- Novosibirsk State University, Pirogova 2, Novosibirsk
- Institute of Automation and Electrometry, Siberian Branch of the Russian Academy of Sciences, Koptyuga 1, Novosibirsk, Russia
*Corresponding author’s E-mail: egik_00@mail.ru
Abstract
Background
Intracellular lipids are sensitive to freezing. Lipidome modification is an important tool for studying the role of intracellular lipids in cryotolerance of mammalian oocytes and preimplantation embryos.
Objective
To study the effects of in vitro exposure of murine embryos to saturated stearic acid (SA) on the lipid content, embryo development and cryotolerance.
Materials and methods
In vivo derived mouse embryos were cultured with 100 µM SA for 48 h up to the morula/blastocyst stage. Some of the SA-treated embryos were chosen for the evaluation of their development competence and the change in the lipidome, and other embryos were either slowly frozen or rapidly vitrified.
Results
Nile red staining combined with confocal laser scanning microscopy revealed a decrease in the total amount of lipids in the SA-treated embryos. Raman measurements showed that the lipid unsaturation was lower in embryos after in vitro SA culture. The addition of SA did not affect the embryo development before cryopreservation, but negatively affected the results of slow freezing cryopreservation and vitrification.
Conclusion
In vitro SA exposure lowered the total amount of intracellular lipids and unsaturation in mouse embryos. The changes were accompanied with a significantly lower efficacy of embryo cryopreservation.
Keywords: confocal microscopy; embryo cryopreservation; lipids; Raman spectroscopy; stearic acid
CryoLetters 45 (1), 36-40 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110712
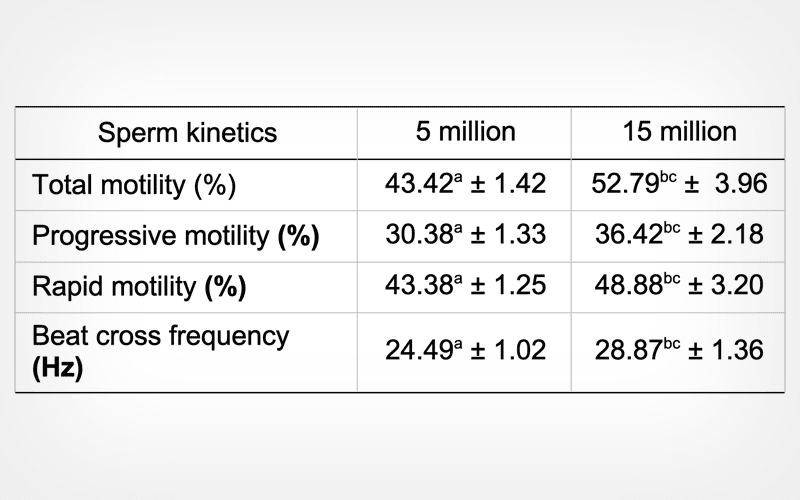
Reduced sperm number of murrah (Bubalus bubalis) bull semen in French mini-straw affects kinetic and functional competence after cryopreservation
HP Yadav1, TK Mohanty1, RK Dewry1, SA Lone1*, S Nath1, M Bhakat2, RK Baithalu3, S Tiwari1, DK Swain4, P Kumar5, AK Mohanty6 and TK Datta7
- Artificial Breeding Research Centre (ABRC), Animal Reproduction, Gynaecology and Obstetrics
- ABRC, Livestock Production Management
- Animal Reproductive Technology Laboratory, Animal Biotechnology Centre, ICAR-NDRI, Karnal, Haryana, India
- Department of Veterinary Physiology, CVSc & AH, U.P. Pandit Deendayal Upadhayaya Pashu Chikitsa Vigyan Vishwavidyalaya Evam Go Anusandhan Sansthan, Mathura, Uttar Pradesh, India
- Animal Physiology and Reproduction Division, ICAR-Central Institute for Research on Buffaloes, Hisar, Haryana, India
- Proteomics Laboratory, Animal Biotechnology Centre
- Animal Genomics Laboratory, Animal Biotechnology Centre, ICAR-NDRI, Karnal, Haryana, India
*Corresponding author’s E-mail: drloneshabir@gmail.com
Abstract
Background
Extensive dilution of cattle semen with tris-based extender compromises certain sperm kinetic and functional traits following cryopreservation.
Objective
To study sperm functions of buffalo bulls under high dilution rates.
Materials and methods
Twenty-four ejaculates were harvested twice a week from four buffalo bulls, and diluted to sperm concentrations of 80, 60, 40 and 20 million/mL. Diluted samples were filled in straws, equilibrated at refrigeration temperature for 4 h, and frozen in liquid nitrogen. Frozen sperm samples were thawed for evaluation of kinetic and functional attributes.
Results
Compared to 20 million/mL (million/mL) sperm sample, the total motility, progressive motility and rapid motility were reduced (P < 0.05) in 5 million/mL sample. The proportion of live sperm were significantly (P < 0.05) higher in 10, 15 and 20 million/mL samples than in 5 million/mL sample. The percentage of moribund sperm, dead sperm, and sperm with lipid per oxidation increased significantly (P < 0.05) in 5 million/mL sample.
Conclusion
The reduction of sperm concentrations to < 10 million/mL affects post-thaw Buffalo sperm kinetic and functional attributes.
Keywords: buffalo bull; cryopreservation; lipid peroxidation; semen dilution; sperm kinetics
CryoLetters 45 (1), 41-48 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110212
Supplementation of extender with chamuangone-enriched Garcinia cowa leaf extract improves quality parameters and longevity of cold-stored cat semen
Saritvich Panyaboriban1, Navapol Kupthammasan1, Kanapot Madsri1, Nattina Mukem1, Sasawan Tarngwiriyakul1, Pokchon Khirilak1, Pharkphoom Panichayupakaranant2,3
and Manita Wittayarat1*
- Faculty of Veterinary Science, Prince of Songkla University, Songkhla, 90110, Thailand
- Department of Pharmacognosy and Pharmaceutical Botany, Prince of Songkla University, Songkhla, 90110, Thailand
- Phytomedicine and Pharmaceutical Biotechnology Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla, 90110, Thailand
*Corresponding author’s E-mail: manita.w@psu.ac.th
Abstract
Background
Semen preservation by cooling is less expensive, simpler and results in less sperm damage than freezing does. However, spermatozoa can only be preserved for a short period due to the excessive formation of reactive oxygen species (ROS). Although several antioxidants can protect sperms from ROS damage during storage at low temperatures, the use of natural antioxidants derived from plants would be a better alternative.
Objective
To assess the effects of chamuangone, which can reduce oxidation reactions in cells, on cat semen quality after preservation at 4°C for 15 days.
Materials and methods
Epididymal sperm samples were collected before being diluted with tris-citric-fructose-egg yolk (TCFE) extender containing different concentrations of chamuangone (0, 50, 100, 150 and 200 μg/mL) and preserved at 4°C. Semen samples were evaluated before chilling and then every 3 days after chilling for up to 15 days. Each sample was assessed for sperm motility, viability, DNA integrity, plasma membrane integrity and percentage of spermatozoa with intact acrosomes.
Results
A significantly higher sperm motility was observed in the group supplemented with 100 μg/mL chamuangone compared to the control after 6 days of storage. However, the chamuangone concentration at 200 μg/mL did not significantly increase the sperm motility when compared to the control for the entire storage period.
Conclusion
100 μg/mL chamuangone can improve sperm characteristics during 15 days of preservation at 4°C, keeping sperm alive (49.3 ± 5.2%) and moving (7.1 ± 2.4%). These results can be used for the development of breeding programs using technologically advanced reproductive procedures in domestic and wild cats.
Keywords: cat semen; chamuangone; cold storage; Garcinia cowa leaf; natural antioxidants
CryoLetters 45 (1), 49-54 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110412
Comparison of different freezing methods for micro-volume semen
Liu Ji1,2*#, Li Yan-Hong1,2#, Zhou Yan-Hua1,2, Wang Xiao-Xiao1,2, Tong Ling-Xi1,2 and Wang Hong-Hui1,2*
- Center of Reproductive Medicine, The Affiliated Weihai Second Municipal Hospital of Qingdao University, Weihai, China
- Center of Reproductive Medicine, Weihai Maternal and Child Health Hospital, Weihai, China
*Corresponding authors’ E-mail: lljj361@163.com and honghui1223@163.com
#These authors contributed equally to this work
Abstract
Background
Micro-volume semen freezing is essential and used popularly for fertility preservation of patients suffering cancer or undergoing male reproductive system related surgeries, and for other reasons that may risk fertility potential in ART cycles. However, clinicians and embryologists still face some unresolved technical and theoretical issues about the frozen-thawed efficiency.
Objective
To choose the appropriate freezing method for different volumes of normal semen samples.
Materials and methods
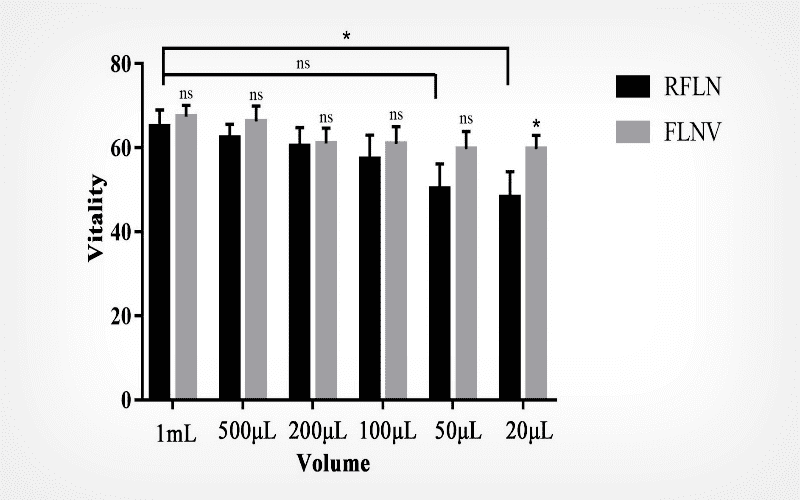
We investigated the frozen-thawed outcomes of semen with different volumes (20 μL, 50 μL, 100 μL, 200 μL, 500 μL and 1 mL) using two freezing methods (FLNV, static liquid nitrogen vapour cooling followed by liquid nitrogen preservation; RFLN, direct rapid freezing in liquid nitrogen) and analyzed the vitality, progressive motility and DNA fragmentation index of thawed sperm.
Results
We found that semen freezing with volumes more than 100 μL had better outcomes than volumes less than or equal to 50 μL after thawing. FLNV presented a higher efficiency for cryopreservation of semen with volumes less than 50 μL.
Conclusion
For smaller (micro) volumes, the FLNV technique is better than the RFLN method.
Keywords: DNA fragmentation; semen cryopreservation; semen volume; sperm quality
CryoLetters 45 (1), 55-59 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110612
Viscoelastic properties of decellularized and freeze-dried human dermis between -90°C and 40°C
Qipeng Jia1, Senli Huang2, Yuting Tang2 and Wendell Q. Sun1*
- Institute of Biothermal Science and Technology, School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai, China
- Beijing Ruijian Gaoke Biotechnology Corporation, Beijing, China
*Corresponding author’s E-mail: wendell.q.sun@gmail.com
Abstract
Background
Human donor skin is processed to make the acellular dermis matrix (ADM) for tissue repair and regeneration. There is no data on the viscoelastic properties of ADM at room and subzero temperatures.
Objective
The work evaluated the temperature dependence of viscoelastic properties of freeze-dried ADM.
Materials and methods
Donor skin was de-epidermized, de-cellularized and freeze-dried with trehalose as the lyo-protectant. Glass transition of freeze-dried ADM was measured by differential scanning calorimeter (DSC), and viscoelastic properties were examined by dynamic mechanical analyzer (DMA).
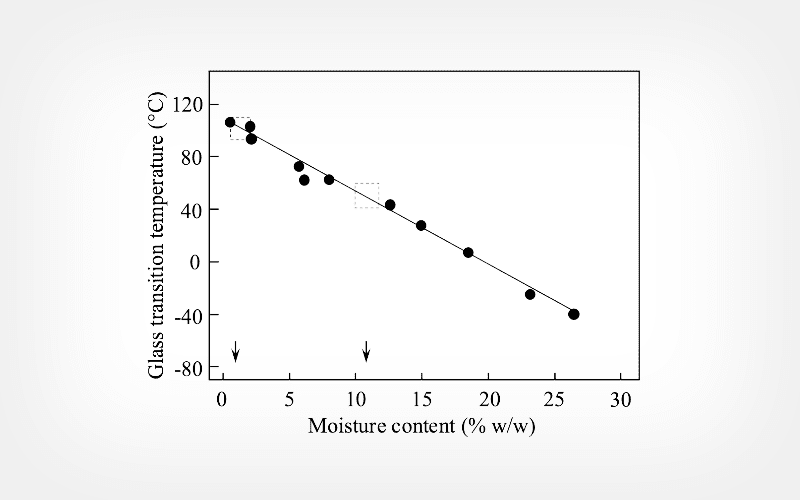
Results
At the low moisture range (1.4 ± 0.5%), the glass transition temperature (Tg) of freeze-dried ADM was 90°C to 100°C. As the moisture content increased, the Tg decreased steadily. At the high moisture range (10.8 ± 2.9%), the Tg was 40°C to 60°C. There were large donor-to-donor variations in viscoelastic properties of freeze-dried ADM as demonstrated by the changes in storage modulus (G’), loss modulus (G”) and damping factor tan δ (G”/G’). However, the trends of the temperature dependence for G’, G” and tan δ were similar among all 8 donors. For each donor, changes in G’ and G” were relatively small between -90°C and 40°C, and G’ was at least one order of magnitude greater than G”. Two viscoelastic relaxations were observed in freeze-dried ADM, one at -20°C and the other at -60°C respectively.
Conclusion
Freeze-dried ADM was protected in the glassy carbohydrate matrix. DMA observed two viscoelastic relaxations (i.e., α process at -20°C and β process at -60°C). Overall changes in G’ and G’’ of freeze-dried ADM were relatively small within one order of magnitude between -90°C and 40°C.
Keywords: acellular human dermis; freeze-drying; glass transition; viscoelastic property
CryoLetters 45 (1), 60-68 (2024)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr24110110812
The effect of different concentrations of laminarin on the quality of cryopreserved ram semen
Nahid Zangishhi, Hadi Hajarian, Hamed Karamishabankareh and Leila Soltani*
- Department of Animal Science, Faculty of Agricultural and Engineering Science, Razi University, Kermanshah, Iran
*Corresponding author’s E-mail: leilasoltani7@yahoo.com
Abstract
Background
Increasingly, sheep breeders are using artificial insemination to produce lambs, so finding methods that preserve ram sperm can be useful.
Objective
To determine the protective effects of different concentrations of laminarin on ram sperm motility, viability, abnormalities, membrane, and DNA integrity, superoxide dismutase enzyme (SOD) activity, and malondialdehyde (MDA) production after freeze-thawing.
Materials and methods
The ejaculates of four rams were collected and stored at 35°C. Semen samples were diluted with a tris-base extender containing 100, 200, 400, and 800 μg/mL of laminarin and a control extender containing no laminarin, then frozen in liquid nitrogen after 4 h in the refrigerator.
Results
In the treatment of frozen-thawed spermatozoa with 800 μg/mL laminarin, motility, viability, membrane integrity, and DNA integrity were significantly higher than in the control. In spermatozoa that were exposed to 800 μg/mL laminarin after thawing, MDA production was significantly lower than in the control group. The percentage of abnormal spermatozoa in 800 μg/mL laminarin was significantly lower than that in the control.
Conclusion
The addition of 800 μg/mL laminarin to the freezing extender increases motility, viability, SOD activity, and plasma membrane integrity, while reducing abnormality and MDA production in freeze-thawed ram semen.
Keywords: cryopreservation; laminarin; ram; semen