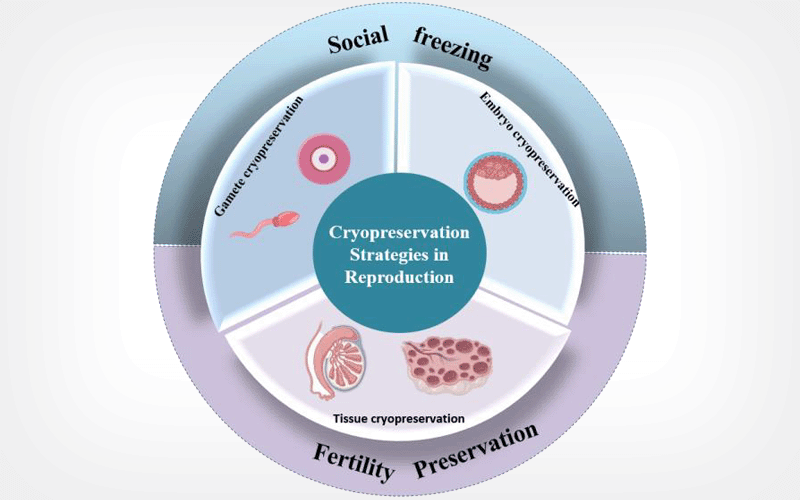
CryoLetters Volume 44 - Issue 4
CryoLetters 44 (4), 185-196 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110112
PERSPECTIVE: Cryobiology and fertility preservation: a perspective on past, current and future studies
Naeimeh Sadat Abtahi1, Zeinab Ghezelayagh1, Iran Nemati1, Farideh Eivazkhani1, Parvaneh Farzaneh2,3, Abdolhossein Shahverdi1, Gholam Reza Goudarzi4, Abdurrahim Pedram5, Elham Amirchaghmaghi6,7, Mojtaba Rezazadeh Valojerdi1,8, Sherman Silber9 and Rouhollah Fathi1,3*
- Department of Embryology, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran
- Human and Animal Cell Bank, Iranian Biological Resource Center (IBRC), ACECR, Tehran, Iran
- Futures Studies Office, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
- Department of Industrial Management, Faculty of Management, Imam Sadiq University, Tehran, Iran
- Futures Studies Office, Supreme National Defense University, Babaei Highway, Tehran, Iran
- Department of Regenerative Biomedicine, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran
- Department of Anatomy, Faculty of Medical Science, Tarbiat Modares University, Tehran, Iran
- Infertility Center of St. Louis, 224 South Woods Mill Road, Suite 730, Saint Louis, MO, 63017, USA
*Corresponding author’s E-mail: rfathi79@royaninstitute.org
Abstract
Cryopreservation has been used over many decades for the maintenance of viable biological specimens. Its expansion into the area of fertility preservation has been a natural outcome of the increased risks to human fertility from diseases, such as cancer and its treatment protocols, including radiation and chemo-therapy, and the general lifestyle trend to later marriages. The use of assisted reproductive techniques (ART) in preserving fertility have benefitted significantly from new scientific approaches, such as cryostorage, in which live cells and tissues are stored at low temperatures and revived when necessary. This review focuses on “cryopreservation science monitoring in reproductive biomedicine” to evaluate knowledge, trends, driving forces, impetus, and emerging technologies in order to draw a future roadmap for this field. Our analysis of the field of cryobiology emphasises the significance of strategic planning of cryobiology research to support more its extensive use in therapeutics in the future. The Royan Institute (Tehran, Iran) recognises this need and has developed a strategic plan to engage in multidisciplinary research on the application of cryobiology, including cryobioengineering, in disease mitigation. We hoped that this study can help improve the quality and quantity of public discourse and expert awareness of the role for cryopreservation in fertility preservation within ART.
Keywords: cryobiology; cryopreservation; fertility preservation; reproduction
CryoLetters 44 (4), 197-207 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110312
Comparative seed cryopreservation of Indonesian and New Zealand epiphytic and terrestrial orchids
Surya Diantina1,2,3*, Craig McGill1, Andrea Clavijo McCormick1, James Millner1, Hugh W. Pritchard5,6 and Jayanthi Nadarajan4*
- School of Agriculture and Environment, Massey University, Tennent Drive, 4410 Palmerston North, New Zealand
- Indonesia Agency for Agricultural Research and Development (IAARD), Jl. Ragunan no. 29 Pasar Minggu, 12540, Jakarta Selatan, Indonesia
- Research Center for Plant Conservation, Botanic Garden and Forestry, National Research and Innovation Agency, Jl. Raya Jakarta-Bogor KM 46, Cibinong, Bogor, 16911, West Java, Indonesia (https://orcid.org/0000-0002-7894-2933)
- The New Zealand Institute for Plant and Food Research Limited, Batchelar Road, Fitzherbert, 4474 Palmerston North, New Zealand
- Royal Botanic Gardens, Kew, Wakehurst, Ardingly, West Sussex RH17 6TN, UK
- Chinese Academy of Sciences, Kunming Institute of Botany, 132 Lanhei Road, Heilongtan, Kunming, Yunnan 650201, China. (https://orcid.org/0000-0002-2487-6475)
*Joint corresponding authors’ E-mails: surya.diantina@brin.go.id or suryadiantina@gmail.com and jayanthi.nadarajan@plantandfood.co.nz
Abstract
Background
The atypical seed storage behaviour reported in several orchid species justifies cryopreservation as a complementary conservation strategy to conventional seed banking.
Objective
This study aimed to assess the seed cryopreservation potential of five orchid species; two tropical epiphytic, Indonesian species (Dendrobium strebloceras, D. lineale), one temperate epiphytic, New Zealand species (D. cunninghamii) and two temperate terrestrial, New Zealand species (Pterostylis banksii, Thelymitra nervosa).
Materials and methods
Seeds were cryopreserved by direct immersion in liquid nitrogen (LN) and through the application of a cryoprotectant vitrification method. For the latter, seeds were exposed to Plant Vitrification Solution 2 (PVS2) for 0, 20, 50, and 70 min, at either room temperature or on ice, prior to immersion in LN.
Results
Seeds of all the studied species germinated well following direct cooling in LN. There was no difference in the seedling development capability between cryopreserved and non-cryopreserved seeds of both tropical epiphytic species and direct immersion in LN enhanced seed germination and shoot formation in both temperate terrestrials.
Conclusion
Through a range of analyses of germination and post-germination growth, our study shows the potential for cryopreserving epiphytic or terrestrial orchids from tropical and temperate regions.
Keywords: cryobiotechnology; ex situ conservation; direct cooling; seed storage; temperate species; tropical species; vitrification

CryoLetters 44 (4), 208-218 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110512
Supplementation of sulfate polysaccharides in the seminal cooling medium of common curimatã (Prochilodus brevis)
Yara Silvino Sales1*, Jéssica Sales Lobato1, Carla Tatiana Nascimento Sousa Vieira1, João Eudes Faria Cavalcante Filho1, Yasmim Maia Ferreira1, Marcos Luiz da Silva Apoliano1, Renata Vieira do Nascimento2, Silvio Alencar Cândido Sobrinho3, José Ariévilo Gurgel Rodrigues3, Carla Pamela Braga Guia4, Fernanda Vitória Almeida Magalhães4 and Carminda Sandra Brito Salmito-Vanderley1
- Laboratório de Andrologia, Departamento de Reprodução Animal, Universidade Federal Rural de Pernambuco. Recife, Brasil
- Laboratório de Bioquímica de Proteínas, Departamento de Bioquímica, Universidade Federal de Pernambuco, Recife, Brasil
- Laboratório de Microbiologia e Biologia Celular, Departamento de Microbiologia, Instituto Aggeu Magalhaes - Fiocruz, Recife, Brasil
*Corresponding author’s E-mail: yara.sales@aluno.uece.br
Abstract
Background
The use of sulfated polysaccharides (PS) in seminal cooling is known to improve seminal quality.
Objective
To evaluate the effect of different concentrations of PS, extracted from the macroalgae Gracilaria domigensis as a supplement to the seminal cooling medium of the reophilic fish Prochilodus brevis (common curimatã).
Materials and methods
Five semen pools were diluted in ACP-104 (treatment T1), in BTS® (T2) and in BTS® with different concentrations of PS (0.5 [T3]; 1.0 [T4] and 1.5 [T5]). The samples were cooled for different times (0, 6, 24, 48, 72, 96 and 120 h) and after each hour they were analyzed for: morphology, membrane integrity, DNA integrity and sperm kinetics.
Results
There were no significant differences between the treatments containing different concentrations of sulfated polysaccharides. Regarding the different cooling times, it was possible to observe that after hour 96, there was a reduction in the parameters of sperm kinetics. For DNA integrity there was no significant difference in relation to the treatments nor in relation to the hours. For membrane integrity, a reduction was noted as of hour 96, but there was no influence of polysaccharides. For the sperm morphology, there was no statistical difference between the hours, however the BTS was better than the ACP-104.
Conclusion
It is concluded that the use of polysaccharides in seminal cooling has no negative effect on sperm parameters and proves that seminal cooling keeps the material viable for up to 72 hours.
Keywords: antioxidants; oxidative stress; reproduction; seaweeds; sperm kinetics

CryoLetters 44 (4), 219-228 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110712
Cryopreservation of zygotic embryos of Podophyllum hexandrum Royle, an endangered medicinal plant, by vitrification and V cryo-plate techniques
Kiran Parasher1, Shailika Sharma1, Papiya Mukherjee1* and Parvaiz Hassan Qazi2
- Department of Botany, Panjab University, Chandigarh, 160014, India
- Plant Molecular Biology and Biotechnology Division, CSIR - Indian Institute of Integrative Medicine, Sanatnagar, Srinagar, 180016, Jammu and Kashmir, India
*Corresponding author’s E-mail: pmukherjee.pu@gmail.com or pmukherjee@pu.ac.in
Abstract
Background
Podophyllum hexandrum is a highly endangered valuable medicinal plant of the Himalayas belonging to family Berberidaceae. This plant needs conservation efforts due to the over-exploitation and unscrupulous harvesting from the wild because of its ever-increasing demand.
Objective
To establish a long-term cryopreservation method for Podophyllum hexandrum using two techniques: Vitrification and V Cryo-plate.
Materials and methods
Zygotic embryos were cryopreserved using vitrification and V cryo-plate by optimization of parameters including preculture time, loading time and PVS2 dehydration time. Recovery of zygotic embryos was performed on different regrowth media for plantlet formation.
Results
With V cryo-plate, 90% regrowth was obtained as compared to 73.3% with vitrification. V Cryo-plate conditions were pre-culture of zygotic embryos in 0.3 M sucrose for 4 days, treatment in loading solution with 0.8 M sucrose for 20 min, dehydration in PVS2 for 50 min, LN exposure, unloading in 1.2 M sucrose for 20 min and transfer of zygotic embryos to regrowth medium for recovery. During recovery, the maximum number of shoots (4.2) and highest shoot length (5.1 cm) were observed on regrowth medium with 1.5 mg/l BAP and 0.1 mg/l IAA (R7).
Conclusion
Zygotic embryos of Podophyllum hexandrum were cryopreserved with 90% regrowth using a V cryoplate technique and plantlets were produced directly after cryopreservation.
Keywords: Podophyllum hexandrum; vitrification; V cryo-plate; zygotic embryos

CryoLetters 44 (4), 229-233 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110212
Strip pulled straw: cost effective alternative cryodevice for the vitrification of oocytes
Khursheed Ahmad Sofi* and Beenish Qureshi
- Division of Veterinary Clinical Complex, F.V.Sc and A.H, Shuhama, SKUAST-Kashmir, Srinagar-190006, India
*Corresponding author’s E-mail: drsofi.vet54321@gmail.com
Abstract
Background
Increased cooling and warming rates by using a suitable cryodevice allows the use of lower cryoprotectant concentration and reduces cryoinjuries as a result of the rapid passage through the ‘dangerous’ temperature zone.
Objective
To evaluate the effectiveness of newly customized strip pulled straw (SPS) with respect to post warming quality, viability, and in vitro maturation for immature oocytes post-vitrification of.
Materials and methods
SPS was prepared using conventional French mini straw to combine the merits of OPS and the Cryotop system. Immature sheep oocytes were treated in 15% EG + 15% DMSO, loaded on SPS and plunged into liquid nitrogen (LN). Post thaw quality, viability, and maturation rates of oocytes were determined after 1 week storage in LN.
Results
SPS achieved a post-thaw morphological survival of 90.9% with 9.0% morphological defects, 96.4% viability and 51% in vitro maturation. In comparison to OPS, SPS had higher post thaw survival (86.5% vs 67.9%) and maturation rate (57.7% vs 50.5%) with lower morphological defects (13.5% vs 32.1%). Cumulus cell loss was the highest among morphological abnormalities of post warm oocytes in SPS (40.9%) and OPS (44.1%). The data showed acceptable post thaw survival, viability and in vitro maturation rate of immature ovine oocytes using SPS as compared to traditional OPS.
Conclusion
SPS can be used as a cost effective alternative device for oocyte vitrification.
Keywords: cryodevice; oocyte; strip pulled straw; vitrification

CryoLetters 44 (4), 234-239 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110412
Freezing of equine semen is influenced by exposure time and concentration of the cryoprotectant glycerol
Pablo Luis Fracaro1, Carine Dahl Corcini2,3*, Norton Luis Souza Gatti1, Jorge Squeff Filho1, Izani Bonel Acosta1, Jorge Squeff Filho1, Felipe Pires Hartwig1, Bruna Da Rosa Curcio1 and Antonio Sergio Varela Junior2,3
- Programa de pós graduação em Veterinária, Faculdade de Veterinária, Universidade Federal de Pelotas, Pelotas, RS, Brasil
- Reprodução Animal Comparada - RAC, Instituto de Ciências Biológicas, Universidade Federal do Rio Grande, Rio Grande, RS, Brasil
- ReproPel, Faculdade de Veterinária, Universidade Federal de Pelotas, Pelotas, RS, Brasil
*Corresponding author’s E-mail: corcinicd@gmail.com
Abstract
Background
Glycerol is a cryoprotectant widely used in the freezing of mammalian species’ semen, but no study has demonstrated its optimum concentration and the appropriate exposure time for equine species.
Objective
To demonstrate that the exposure time (15, 30, 45, 60, 75 and 90 min) versus concentration (2, 3, 4 and 5%) of the cryoprotectant glycerol influences the freezing success of equine semen.
Materials and methods
The ejaculate of 12 stallions were frozen in different glycerol concentrations following different exposure times. The thawed sperm was evaluated for kinetic parameters using a Computer Assisted Semen Analysis (CASA) system and cell feature parameters were assessed by flow cytometry.
Results
Considering the total and progressive motility of the spermatozoa, we concluded that protocols using 5% glycerol for 15 and 30 min exposure, 4% glycerol for 45 min exposure and 3% glycerol for 90 min exposure generated the best results.
Conclusion
We suggest the use of any of these protocols for a better cryopreservation of equine semen.
Keywords: conservation; sperm; equine; motility
CryoLetters 44 (4), 240-248 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23410110612
In vitro cold acclimation is required for successful cryopreservation of Juglans regia L. shoot tips
Svetlana V. Kushnarenko*, Nazgul K. Rymkhanova, Moldir M. Aralbayeva and Natalya V. Romadanova
- Institute of Plant Biology and Biotechnology, Timiryazev st 45, Almaty 050040, Kazakhstan
*Corresponding author’s E-mail: svetlana_bio@mail.ru
Abstract
Background
Persian walnut (Juglans regia L.) is one of the most economically important nut crops. In the Western Tien Shan in Kazakhstan, walnut forests are the northernmost in the natural range of this species. Shoot tip cryopreservation is an important strategy to ensure long-term clonal conservation of plant genetic resources.
Objective
To determine the effect of cold acclimation duration (0-6 weeks) with alternating temperatures (8 h at 22°C, light intensity 10 μmol m-2 s-1/16 h at -1°C, in the dark) and of plant vitrification solution 2 (PVS2) exposure duration (30, 50, 80 or 100 min) on shoot tip regrowth after cryopreservation by vitrification.
Materials and methods
In vitro-grown shoots of three wild Juglans regia accessions from a native population of Sairam-Ugam National Natural Park in the south of Kazakhstan and of cultivar Milotai 10 were used as sources of plant material. Shoot tips (1.8-2.0 mm with five to six leaf primordia) were excised from in vitro-grown shoots and cryopreserved using PVS2 vitrification technique.
Results
Regrowth of cryopreserved shoot tips increased and was significantly higher (P < 0.05) after 4-6 weeks cold acclimation with the highest regrowth between 59.9-67.8% after 5 weeks for the four genotypes tested. The highest regrowth level was obtained after 80 min of PVS2 exposure, which was significantly better (P < 0.05) compared to 30, 50 or 100 min exposure.
Conclusion
The PVS2 vitrification protocol established is very effective for cryopreservation of both unique wild J. regia germplasm and of walnut cultivars.
Keywords: long-term storage; pretreatment; plant vitrification solution; shoot tips; vitrification; walnut