CryoLetters Volume 44 - Issue 3
CryoLetters 44 (3), 123-133 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110112
PERSPECTIVE: The role of cryopreservation techniques in manufacturing, transport, and storage of CAR-T therapy products
Miroslava Jandová1,2*, Glyn Nigel Stacey3,4,5, Miriam Lánská6, Jiří Gregor1, Petra Rozsívalová7, Lenka Beková7, Zuzana Woidigová Ducháčová7, David Belada6, Jakub Radocha6, Pavel Měřička1 and Barry Fuller8
- Department of Histology and Embryology, Faculty of Medicine in Hradec Králové, Charles University, Czech Republic
- Department of Histology and Embryology, Faculty of Medicine in Hradec Králové, Charles University, Czech Republic
- International Stem Cell Banking Initiative, 2 High Street, Barley, Herts, SG88HZ, UK
- National Stem Cell Resource Centre, Institute of Zoology, Chinese Academy of Sciences, Beijing 100190, China
- Institute for Stem Cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China
- 4th Department of Internal Medicine - Haematology, Faculty of Medicine in Hradec Králové, Charles University and University Hospital Hradec Králové, Czech Republic
- Hospital Pharmacy, University Hospital Hradec Králové, Czech Republic
- Division of Surgery, UCL and Royal Free London NHS Trust, London NW3 2QG
*Corresponding author’s E-mails: jandomir@fnhk.cz
Abstract
Several clinical trials have proved the efficacy and safety of T-cells chimeric antigen receptor (CAR-T cells) in treatment of malignant lymphoma and the first products were registered in the European Union in 2018. The shelf-life of CAR-T cell products in the liquid state is short, so cryopreservation offers a significant benefit for logistics in manufacturing and patient management. Direct shipment of the cryopreserved CAR-T cell therapy products to the clinical department is feasible, nevertheless, intermediate storage in the hospital cryostorage facility gives significant advantage in planning of their administration to patients. Moreover, some manufacturers prefer transport of the starting material cryopreserved at the collection site. The cryopreservation protocol used for starting material by the authors is based on combining dimethyl sulphoxide (DMSO) with hydroxyethyl starch (HES) and slow controlled cooling in cryobags housed in metal cassettes. This achieves the mononuclear cell post-thaw viability of 98.8 ± 0.5 % and recovery of 72.8, ± 10.2 %. Transport of the starting material to the manufactures and return transport of the CAR-T therapy product is performed by authorized courier companies. Intermediate cryostorage of the final CAR-T cell therapy product is performed in a separate dry-storage liquid nitrogen container. On the day of infusion, the cryopreserved products are transported to the clinical department in a dry shipper. On the wards the product is removed from the cassette, inserted into a sterile plastic bag, thawed in a 37 °C water bath followed by immediate intravenous administration. The authors discuss the adherence of the used technology to good manufacturing practice (GMP) principles and genetic safety assurance rules.
Keywords: CAR-T, advanced therapy medicinal product, cryopreservation

CryoLetters 44 (3), 134-141 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110312
Effect of isolation protocols and cryoprotectants on freezing of stallion epididymal spermatozoa
T. R. Talluri1*, D. Jhamb2, Nilendu Paul3, J. Singh1, R. K. Dedar1, S. C. Mehta1, R. A. Legha1 and Yash Pal4
- ICAR- National Research Centre on Equines, Equine Production Campus, Bikaner, Rajasthan-334001, India
- College of Veterinary and Animal Sciences, Navania, Udaipur, Rajasthan, India
- ICAR-Central Institute for Research on Buffaloes, Nabha, Punjab, India
- ICAR- National Research Centre on Equines, Hisar, Haryana-125001, India
*Corresponding author’s E-mail: raotalluri79@gmail.com or Talluri.Rao@icar.gov.in
Abstract
Background
The recovery of spermatozoa from the cauda epididymis may be the only option to obtain genetic material from elite stallions that had undergone castration or sudden death due to colic or severe injury.
Objective
To evaluate two different protocols for retrieval of stallion epididymal spermatozoa and to evaluate different cryoprotectants on the freezability of the epididymal spermatozoa.
Materials and methods
Six epididymides from three stallions were collected immediately after routine castration under general anesthesia. In the first experiment, each epididymis (of two testes) of the same stallion were processed using different methods for retrieval of the epididymal spermatozoa and were pooled and cryopreserved either using 5% glycerol or 5% dimethyl formamide (DMF) as cryoprotectant. The semen quality parameters viz., progressive motility, HOST, viability and acrosome integrity were evaluated at the fresh, pre-freeze and post-thaw stages.
Results
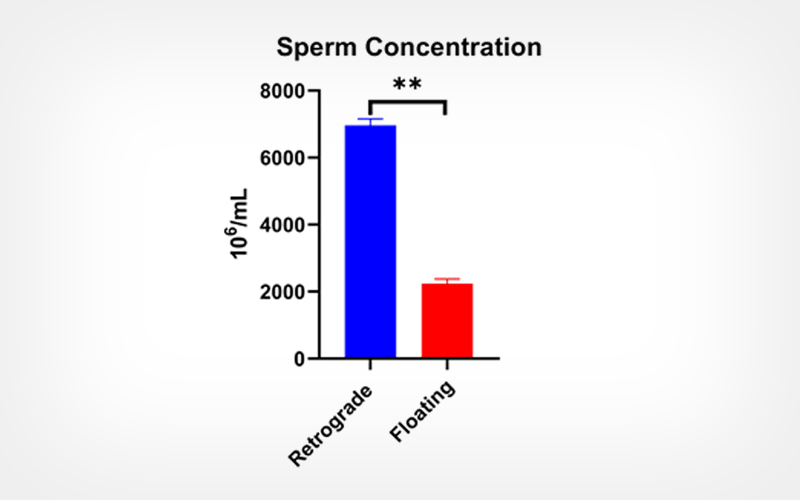
Retrograde method of flushing of epididymis yielded significantly (p < 0.05) higher concentration of the stallion sperm than that of the floating method. The qualitative semen parameters i.e., viability, plasma membrane integrity and acrosome integrity were found to be significantly restored using 5% DMF as cryoprotectant in comparison to when  5% glycerol was used.
Conclusion
Retrograde flushing method of epididymis yielded significantly higher sperm concentration to that of the floating method, and 5% DMF as cryoprotectant provided acceptable freezability of stallion epididymal spermatozoa.
Keywords: cryoprotectant; DMF; epididymal sperm; glycerol; stallion
CryoLetters 44 (3), 142-150 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110512
Comparative cryopreservation of Indian wild orange (Citrus indica Tanaka) embryonic axes
S.K. Malik1,*, Sukhdeep Kaur1, Ravish Choudhary2, Rekha Chaudhury1 and Hugh W. Pritchard3,4
- Tissue Culture and Cryopreservation Unit, National Bureau of Plant Genetic Resources, New Delhi-110012
- Division of Seed Science and Technology, Indian Agricultural Research Institute, New Delhi-110012
- Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650201, P.R. China. ORCID: 0000-0002-2487-6475
- Royal Botanic Gardens, Kew, Wakehurst, Haywards Heath, West Sussex RH17 6TN, UK
*Corresponding author’s E-mail: skm1909@gmail.com
Abstract
Background
Indian Wild Orange (Citrus indica Tanaka) is an endangered and endemic species from northeast India for which effective ex situ conservation strategies, including embryo cryopreservation, are urgently needed.
Materials and methods
Desiccation tolerance and cryopreservation ability for embryonic axes of Citrus indica was determined using three techniques (air desiccation-freezing, PVS2 vitrification-freezing and encapsulation-dehydration-freezing). Success was assessed as survival and recovery in vitro.
Results
Successful cryopreservation of embryonic axes was achieved using all three methods, with the highest survival achieved when using air desiccation-freezing (90%) followed by encapsulation-dehydration (85%) and PVS2 vitrification cryopreservation (80%). Regeneration levels were lower than survival levels for all three proceedures. Post-cryo regeneration success was: encapsulation-dehydration (64%) > air desiccation-freezing (55%) > PVS2 vitrification (52%).
Conclusion
Although there was relatively high post-cryopreservation recovery growth obtained using all the three techniques, the air desiccation-freezing technique is preferred, as it is a simple, practical and reproducible technique for the long-term cryobanking of this important wild species.
Keywords: air desiccation; encapsulation; plant conservation; pre-culture; vitrification

CryoLetters 44 (3), 151-159 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110712
Aqueous extract of Moringa oleifera Lam. leaves added to freezing extenders damages goat sperm membranes
Desirée Coelho de Mello Seal*1, Millena Maria Monteiro1, Lúcia Cristina Pereira Arruda1, Jerônimo Hugo de Souza1, Robson Raion de Vasconcelos Alves2, Luiz Alberto Lira Soares2, Thiago Henrique Napoleão2, Patrícia Maria Guedes Paiva2, Lucas Eduardo Bezerra de Lima3, Regina Célia Bressan Queiroz de Figueiredo3 and Maria Madalena Pessoa Guerra1
- Laboratório de Andrologia, Departamento de Reprodução Animal, Universidade Federal Rural de Pernambuco. Recife, Brasil
- Laboratório de Bioquímica de Proteínas, Departamento de Bioquímica, Universidade Federal de Pernambuco, Recife, Brasil
- Laboratório de Microbiologia e Biologia Celular, Departamento de Microbiologia, Instituto Aggeu Magalhaes – Fiocruz, Recife, Brasil
*Corresponding author’s E-mail: desiree.seal@hotmail.com
Abstract
Background
Semen cryopreservation is a biotechnology used frequently in animal production; however, there are some obstacles, such as those caused by high levels of reactive oxygen species (ROS). Moringa oleifera (MO) is known as a potent source of antioxidants and might be an important adjuvant.
Objective
The objective of this study was to determine the effect of different concentrations of MO extract supplementation on goat semen cryopreservation efficiency.
Materials and methods
Ejaculates (n=6) from four goat breeders were pooled and diluted in skimmed milk (SM) or Tris-egg yolk (TEY)-based extenders and supplemented with different concentrations of MO extract (0, 1, 2 and 5 mg/mL). After the freeze-thaw cycle, sperm kinetics and viability were assessed.
Results
With the SM extender, straightness, wobble and plasma membrane integrity were lower than in the control group (P < 0.05). With the TEY extender, wobble was lower in with 5 mg/mL MO extract than in the control group (P < 0.05). As regards sperm ultrastructure, evaluated by SEM, the MO extract, regardless of the diluent used, damaged the membrane of sperm cells in a dose-dependent manner.
Conclusion
The addition of aqueous extract of MO leaves in both diluents at all concentrations tested affects the parameters of sperm progressivity and damages the plasma membrane in a dose-dependent manner.
Keywords: antioxidant; natural extract; reactive oxygen species; skimmed milk; tris-egg yolk
CryoLetters 44 (3), 160-168 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110212
Assessing the benefits of antioxidant and antibiotics treatments for cryopreservation of the model alga Ectocarpus siliculosus and the endemic alga Acinetospora asiatica
Jose Avila-Peltroche, Boo Yeon Won and Tae Oh Cho1*
- Department of Life Science, Chosun University, Gwangju 61452, Korea
*Corresponding author’s E-mail: tocho@chosun.ac.kr
Abstract
Background
Cryopreservation in liquid nitrogen is a suitable technique for preserving seaweeds, a group of photosynthetic organisms with many applications. Although there are some standard protocols for seaweed cryopreservation, most rely on expensive controlled-rate coolers. Moreover, several factors, such as the use of antioxidants or antibiotics, remain unexplored.
Objective
To test the effect of 2-mercapthoethanol (antioxidant) and antibiotic mixtures on the cryopreservation of the model alga Ectocarpus siliculosus and the endemic brown seaweed Acinetospora asiatica using a low-tech passive rate cooler.
Materials and methods
2-mercaptoethanol was added to the cryoprotectant (CPA) solution, while antibiotic mixtures were included in the culture medium during the recovery process. In addition, two CPA solutions were tested on E. siliculosus.
Results
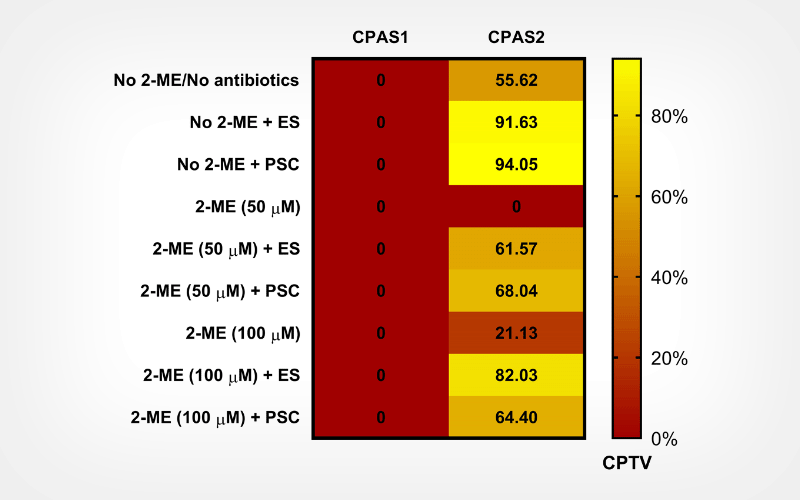
After two weeks of recovery, the treatment comprising PSC antibiotic mixture (Penicillin G, streptomycin, and chloramphenicol) showed a significant increase in post-thaw viability. Antioxidant treatment did not improve viability. The highest viabilities for E. siliculosus and A. asiatica were 64-83%, and 83-87%, respectively, using 10% glycerol + 10% proline as CPA solution.
Conclusion
E. siliculosus and A. asiatica were successfully cryopreserved using a low-tech passive rate cooler, 10% glycerol + 10% proline solution, and antibiotic treatment. The highest post-thaw viabilities (64-87%) reported for PSC antibiotic mixture suggest the potential benefits of using antibiotics during post-thaw recovery of marine macroalgae. This study is the first report on the cryopreservation of A. asiatica.
Keywords: Acinetospora; antibiotics; brown algae; cryopreservation; Ectocarpus; passive cooling
CryoLetters 44 (3), 169-177 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110412
Effect of cryoprotectant and pre-freezing on the sperm motility, viability and fertility of goldfish Carassius auratus (Pisces: Cyprinidae) post short-term cryopreservation
Nurlaili Nurlaili1, Kartini Eriani1, Itsnatani Salma1, Siti Maulida2,
Sri Riska Rahayu2, Luvi Syafrida Handayani2,
Filiz Kutluyer Kocabas3, Mohd Nor Siti-Azizah4,
Martin Wilkes5 and Zainal Abidin Muchlisin2*
- Master Program in Biology, Faculty of Matemathics and Natural Sciences, Syiah Kuala University, Banda Aceh, 23111, Indonesia
- Department of Aquaculture, Faculty of Marine and Fisheries, Syiah Kuala University, Banda Aceh, 23111 Indonesia
- Faculty of Fisheries, Munzur University, Turkey
- Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia
- University of Essex, Essex, England
*Corresponding author’s E-mail: muchlisinza@unsyiah.ac.id
Abstract
Background
Goldfish Carassius auratus is a popular ornamental fish extensively cultured worldwide. Sperm cryopreservation is a common fish breeding method that ensures sperm availability around the year. Studies on cryopreservation of goldfish sperm, especially on the suitability of cryoprotectant types and pre-freezing time, are scarcely available.
Objective
To determine the most suitable type of cryoprotectant and pre-freezing for the successful cryopreservation of goldfish sperm.
Materials and methods
A completely randomized design with two factors was utilized in this study. The first factor is the type of cryoprotectants, which included methanol, ethanol, ethylene glycol, glycerol, and DMSO. The second is pre-freezing times of 10, 20, 30, and 40 min at each of the pre-freezing temperatures of 4°C, -10°C, and -79°C, meaning that the total times for the ramping down of temperature were 30, 60, 90 and 120 min, respectively. The Ringer solution and 10% egg yolk were used as extender and extracellular cryoprotectant. The sperm was stored at -179°C for 7 days.
Results
The ANOVA test showed that cryoprotectants and pre-freezing significantly affected the motility, viability, and fertility of goldfish sperm after freezing in liquid nitrogen for 7 days (P<0.05). Furthermore, 10% DMSO combined with 15% egg yolk with an pre-freezing time of 20 min can maintain sperm motility, viability, and fertility higher than other treatments, by 79%, 80%, and 33%, respectively. The agarose gel electrophoresis showed no DNA fragmentation in all samples, including fresh sperm.
Conclusion
We conclude that 10% DMSO combined with 15% egg yolk and 20 min pre-freezing is the best treatment for goldfish sperm cryopreservation.
Keywords: DNA fragmentation; freezing; sperm motility; sperm viability; thawing

CryoLetters 44 (3), 178-184 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23310110612
The safety of human embryos following long-term cryopreservation (≥6 years) on vitrification
Hui He1#, Rui Jiang2#, Xinling Ren1, Lei Jin1* and Yaping Jiang1*
- Department of Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, PR China
- Laboratory of Clinical Immunology, Wuhan No. 1 Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, PR China
*Corresponding author’s E-mail: jiangyp@tjh.tjmu.edu.cn
#Equal contributors
Abstract
Background
Vitrification of embryos has become the basic means of assisted reproductive technology (ART) therapy in recent years. Concerns have also been raised about the safety of vitrification and the effect of cryopreservation time. Most of the previous studies were on the data within 6 years of cryopreservation.
Objective
In this study, we aimed to evaluate the impact of long-term cryopreservation (≥6 years) on pregnancy and neonatal outcomes.
Materials and methods
This research was a single-center, retrospective analysis, including 426 frozen-thawed embryo transfer (FET) cycles. Patients who participated in IVF-FET cycles between January 2013 to December 2020 were analyzed. Preferentially matched participants were divided into three groups according to storage time: group A (≥72 months), group B (0-3 months, propensity score matching [PSM] according to the age of oocyte retrieval), and group C (0-3 months, PSM according to the age of embryo transfer).
Results
Our results revealed that there were no significant differences in human chorionic gonadotropin [HCG] positive rate, clinical pregnancy rate, miscarriage rate, live birth rate, and neonatal outcomes when the embryo storage duration ≥72 months. But the proportion of high birth weight was higher in group A (≥72 months) when matched according to age at embryo transfer.
Conclusion
The results of our study showed that long-term cryopreservation had no effect on the pregnancy and neonatal outcomes of vitrification. The results offer evidence for the safety of using long-term cryopreservation embryos after vitrification.
Keywords: cryopreservation, long term, embryo vitrification, frozen embryo transfer, pregnancy outcome







