CryoLetters Volume 44 - Issue 2
CryoLetters 44 (2), 65-75 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110112
PERSPECTIVE: Cryopreservation of organoids
Olena Rogulska1,2,3*, Jarmila Havelkova2,3 and Yuriy Petrenko2,3*
- Department of Biochemistry, Institute for Problems of Cryobiology and Cryomedicine of the NAS Ukraine, Kharkiv, Ukraine
- Department of Biomaterials and Tissue Engineering, Institute of Physiology of the CAS, Prague, Czech Republic
- Department of Neuroregeneration, Institute of Experimental Medicine of the CAS, Prague, Czech Republic
*Corresponding authors’ E-mails: rogulskaya.elena@gmail.com and yuriy.petrenko@iem.cas.cz
Abstract
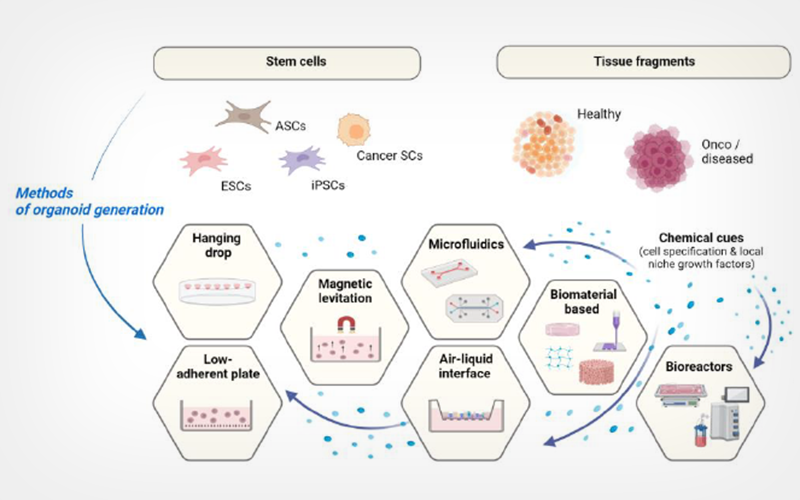
Organoids represent indispensable opportunities for biomedicine, including drug discovery, cancer biology, regenerative and personalised medicine or tissue and organ transplantation. However, the lack of optimised preservation strategies limits the wide use of organoids in research or clinical fields. In this review, we present a short outline of the recent developments in organoid research and current cryopreservation strategies for organoid systems. While both vitrification and slow controlled freezing have been utilized for the cryopreservation of organoid structures or their precursor components, the controlled-rate slow freezing under protection of Me2SO remains the most common approach. The application of appropriate pre- or post-treatment strategies, like the addition of Rho-kinase or myosin inhibitors into cell culture or cryopreservation medium, can increase the recovery of complex organoid constructs post-thaw. However, the high complexity of the organoid structure and heterogeneity of cellular composition bring challenges associated with cryoprotectant distribution, distinct response of cells to the solution and freezing-induced injuries. The deficit of adequate quality control methods, which may ensure the assessment of organoid recovery in due term without prolonged re-cultivation process, represents another challenge limiting the reproducibility of current cryobanking technology. In this review, we attempt to assess the current demands and achievements in organoid cryopreservation and highlight the key questions to focus on during the development of organoid preservation technologies.
Keywords: organoids; stem cells; tumour; cryopreservation; vitrification; recovery
CryoLetters 44 (2), 76-79 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110312
Stabilization of labile active ingredients in an oil-water emulsion cosmetics by freeze-drying
Zuxin Yi, Mei Yang and Baolin Liu
- Institute of Biothermal Science and Technology, University of Shanghai for Science and Technology, 516 Jungong Road, Shanghai 200093, China
*Corresponding author’s E-mail: blliuk@usst.edu.cn
Abstract
Background
Due to the instability in oil/water emulsion, certain labile active ingredients were often not used in cosmetics.
Objective
The present study has tested the effect of freeze-drying to stabilize an oil/water cosmetic emulsion.
Materials and methods
A preliminary freeze-drying process was established at the basis of calorimetric and freeze-drying microscope studies. The stability of labile molecules in the cosmetic emulsion was evaluated at 48°C after freeze-drying.
Results
The accelerated stability experiment showed that the freeze-dried emulsion retained 90.1% vitamin C after 28 days at 48°C, whereas the oil-water emulsion retained only 28.3% vitamin C. The freeze-dried emulsion had significantly less oil oxidation than did the oil-water emulsion.
Conclusion
Freeze-drying improved the stability of vitamin C and oily active ingredients in cosmetic emulsions.
Keywords: cosmetics; freeze drying; oil-water emulsion; biological stabilization

CryoLetters 44 (2), 80-88 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110512
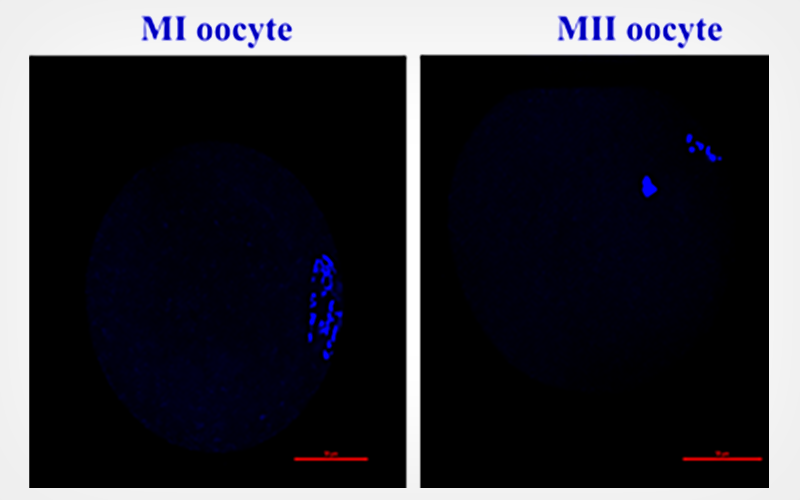
Human follicular fluid and mesenchymal stem cell conditioned medium improves in vitro development of vitrified-warmed mouse oocytes
Shirin Geravandi1#, Eshrat Kalehoei1#, Azadeh Karami2,
Fatemeh Nowrouzi1, Zahra Kalhori1, Hossein Zhaleh3
and Mehri Azadbakht1*
- Department of Biology, Faculty of Basic Sciences, Razi University, Kermanshah, Iran
- Department of Anatomical Sciences and Biology, Faculty of Medicine, University of Medical Sciences, Kermanshah, Iran
- Substance Abuse Prevention Research Center, University of Medical Sciences, Kermanshah, Iran
*Corresponding author’s E-mail: azadbakhtme@gmail.com
#Shared first authors
Abstract
Background
In vitro maturation (IVM) and oocyte cryopreservation are therapeutic options in assisted reproductive technology which is used to preserve fertility in patients with different causes of infertility.
Objective
To analyze in vitro development of vitrified-warmed oocytes in the presence of human follicular fluid (FF) and bone marrow mesenchymal stem cell-conditioned medium (BMSC-CM) as a rescue strategy in fertility preservation.
Materials and methods
BMSC-CM and FF media were used as two natural media. Not only osteogenic and adipogenic differentiation but also flow cytometry was carried out to confirm the nature of mesenchymal stem cells. A total of 327 vitrified-warmed oocytes were randomly assigned to three groups with different maturation media. After 24 h the maturation rate was evaluated. In vitro fertilization and also embryo development were also assessed.
Results
Oocytes matured in the BMSC-CM and FF groups showed a significant increase compared to the control group (76.6±2.9, 53.2±1.0 , and 40.8±6.1, respectively) (P < 0.05). Embryo cleavage rates in the BMSC-CM were dramatically higher than FF and control groups (85.6±2.2, 70.5±2.2, and 60.7±1.5, respectively). Blastocyst formation rates in the BMSC-CM group were statically different compared to FF and control groups (73.6±1.0, 58.5±1.0, and 45.8±4.2, respectively).
Conclusion
BMSC-CM and FF media not only improve the maturation rate of vitrified warmed oocytes but also significantly increase embryo cleavage and blastocyst rates.
Keywords: human follicular fluid; in vitro development; mesenchymal stem cell-conditioned medium; mouse oocytes; vitrification

CryoLetters 44 (2), 89-99 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110712
Camptothecin: solubility, in-vitro drug release, and effect on human red blood cells and sperm cold preservation
Sofiane Fatmi1,2,4, Lamia Taouzinet2,3*, Mohamed Skiba4
And Mokrane Iguer-Ouada2
- Technology Pharmaceutical Laboratory, Department of Processes Engineering, Faculty of Technology, Université de Bejaia, 06000 Bejaia, Algeria
- Associated Laboratory in Marine Ecosystems and Aquaculture, Faculty of Nature and Life Sciences, Université de Bejaia, 06000 Bejaia, Algeria
- Centre de Recherche en Technologies Agro-Alimentaires, Campus Universitaire Tergua Ouzemour, Bejaia 06000, Algeria
- Technology Pharmaceutical and Bio pharmaceutics Laboratory, UFR Medicine and Pharmacy, Rouen University, 22 Blvd. Gambetta, 76183, Rouen, France
*Corresponding author’s E-mail: lamia.taouzinet@univ-bejaia.dz or lamia.taouzinet@crtaa.univ-bejaia.dz ORCID: 0000-0001-8992-1591
Abstract
Background
Camptothecin (CPT) is an anticancer drug, and is not employed in the clinic because of its high hydrophobicity and low active form stability. CPT may also have potential for use in cold preservation.
Objective
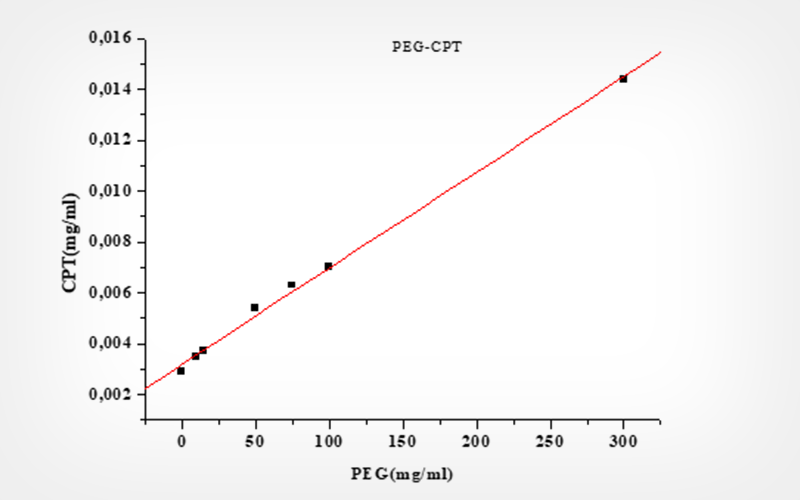
To overcome these drawbacks, CPT solubility variations in the presence of cyclodextrins (CDs) and polyethylene glycol (PEG) were evaluated by Higuchi solubility experiments.
Materials and methods
CPT was encapsulated in different cyclodextrins and polyethylene glycol using a co-evaporation method. The CPT interactions with CDs and PEG 6000 were investigated by Fourier-transformed infrared spectroscopy (FT-IR), and X-ray powder diffraction (XRPD). Then, CPT complexes were evaluated for in-vitro drug release. To evaluate the potential anticancer efficacy of the CPT complexes system, in-vitro cytotoxicity studies on human red blood cells were carried out using UV assay. The impact of the CPT complex systems on sperm motility protection during cold preservation at 4°C was studied using CASA.
Results
The dissolution profile of these preparations shows the improvement of the dissolution of the CPT following a fickien diffusion. The CPT solubility and stability improvement were the cause of the cytotoxicity on the red blood cells test. However, CPT alone, encapsulated, dispersed, and chemically modified protected spermatozoids during cold preservation.
Conclusion
We confirm the interest in CPT encapsulated and dispersed in anticancer treatments. We also found that CPT encapsulated or dispersed could protect sperm against oxidative damage and improve the membrane integrity of human sperm. Consequently, CPT encapsulated our dispersed could eventually be beneficial for infertility therapy.
Keywords: camptothecin; cold storage; cyclodextrins; human semen; PEG 6000; red globule
CryoLetters 44 (2), 100-108 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110212
Interactions among sucrose and concentrations of serum fetal bovine on the cryopreservation of somatic cells derived from red-rumped agouti
Érika Almeida Praxedes, Maria Bárbara Silva, Lhara Ricarliany Medeiros de Oliveira, João Vitor da Silva Viana and Alexsandra Fernandes Pereira*
- Laboratory of Animal Biotechnology, Federal Rural University of Semi-Arid, Mossoro, RN, Brazil
*Corresponding author’s E-mail: alexsandra.pereira@ufersa.edu.br
Abstract
Background
The synergistic action among the different extracellular cryoprotectants could improve somatic cell quality after thawing and provide bases for the formation of biobanks for red-rumped agoutis.
Objective
This study evaluated the interactions among sucrose (SUC) and concentrations of serum fetal bovine (FBS) on the cryopreservation of somatic cells derived from red-rumped agoutis.
Materials and methods
Cells were cryopreserved with 10% dimethyl sulfoxide and different concentrations of FBS (10%, 40%, and 90%) with or without 0.2 M SUC, totaling six comparison groups. Non-cryopreserved cells were used as a control. Cells were evaluated for viability, metabolic activity, proliferative activity, reactive oxygen species (ROS), mitochondrial membrane potential (ΔΨm) and apoptosis levels.
Results
No difference was observed among cryopreserved with DMSO containing (10FBS, 10FBS-SUC, 40FBS, 40FBS-SUC, 90FBS, 90FBS-SUC) and non-cryopreserved groups for viability, metabolic activity, proliferative activity, and ROS levels. Interestingly, only cells cryopreserved with 90% FBS and SUC maintained the ΔΨm like the control. This indicates that at high concentrations of FBS, SUC contributes to the maintenance of this parameter in cryopreserved cells. Moreover, at concentrations of 10% and 40% of FBS, SUC contributed to the maintenance of viability evaluated by the levels of apoptosis evaluated after thawing. In summary, we verified that 90% FBS and 0.2 M SUC promote greater ability of cells after thawing. Additionally, SUC positively acts in cryopreservation solutions containing 10% and 40% FBS.
Conclusion
This information is essential to an understanding of the mechanisms involved in the interactions of extracellular cryoprotectants in somatic cell cryopreservation solutions of red-rumped agoutis.
Keywords: cell bank; Dasyprocta genus; extracellular cryoprotectants; in vitro culture; wild rodent
CryoLetters 44 (2), 109-117 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110412
Effect of antioxidant procyanidin B2 (PCB2) on ovine oocyte developmental potential in response to in vitro maturation (IVM) and vitrification stress
Jiachen Bai1#, Jun Li2#, Longfei Wang3, Shaopeng Hao4, Yanhua Guo4, Yucheng Liu4, Zhenliang Zhang4, Houru Li4, Wendell Q. Sun1, Guoqing Shi4, Pengcheng Wan4* and Xiangwei Fu3,4*
- Institute of Biothermal Science and Technology, School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
- Department of Reproductive Medicine, Reproductive Medical Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, China
- National Engineering Laboratory for Animal Breeding, Beijing Key Laboratory for Animal Genetic Improvement, College of Animal Science and Technology, China Agricultural University, Beijing 100193, China
*Corresponding authors’ E-mail: xiangweifu@126.com and pengcheng.wan@gmail.com
#Equal contributors
Abstract
Background
It was demonstrated that external stress, such as in vitro maturation (IVM) and vitrification process can induce significantly reduced development capacity in oocytes. Previous studies indicated that antioxidants play a pivotal part in the acquisition of adaptation in changed conditions. At present, the role of the natural potent antioxidant PCB2 in response to IVM and vitrification during ovine oocyte manipulation has not been explored.
Objective
To investigate whether PCB2 treatment could improve the developmental potential of ovine oocytes under IVM and vitrification stimuli.
Materials and methods
The experiment was divided into two parts. Firstly, the effect of PCB2 on the development of oocytes during IVM was evaluated. Un-supplemented and 5 μg/mL PCB2-supplemented in the IVM solution were considered as control and experimental groups (C + 5 μg/mL PCB2). The polar body extrusion (PBE) rate, mitochondrial membrane potential (MMP), ATP, reactive oxygen species (ROS) levels and early apoptosis of oocytes were measured after IVM. Secondly, we further determine whether PCB2 could improve oocyte quality under vitrification stress. The survival rate, PBE rate and early apoptosis of oocytes were compared between fresh group, vitrified group and 5 μg/mL PCB2-supplemented in the IVM solution after vitrification (V + 5μg/mL PCB2).
Results
Compared to the control group, adding PCB2 significantly increased PBE rate (79.4% vs. 62.8%, P < 0.01) and MMP level (1.9 ± 0.08 vs. 1.3 ± 0.04, P < 0.01), and decreased ROS level (47.1 ± 6.3 vs. 145.3 ± 8.9, P < 0.01). However, there was no significant difference in ATP content and early apoptosis. Compared to the fresh group, vitrification significantly reduced oocytes viability (43.0% vs. 90.8%, P < 0.01) as well as PBE rate (24.2% vs. 60.6%, P < 0.05). However, 5 μg/mL PCB2-supplemention during maturation had no effect on survival, PBE or early apoptosis in vitrified oocytes.
Conclusion
PCB2 could effectively antagonise the oxidative stress during IVM and promote oocyte development.
Keywords: COCs; IVM; PCB2; sheep; vitrification
CryoLetters 44 (2), 118-122 (2023)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr23210110612
Development and genome mutation of bovine zygotes vitrified before and after genome editing via electroporation
Thanh-Van Nguyen1,3, Lanh Thi Kim Do1,3*, Zhao Namula2,3,
Qingyi Lin3,4, Nanaka Torigoe3,4, Megumi Nagahara3,4,
Maki Hirata3,4, Fuminori Tanihara3 and Takeshige Otoi3,4
- Faculty of Veterinary Medicine, Vietnam National University of Agriculture, 100000 Hanoi, Vietnam
- College of Coastal Agricultural Sciences, Guangdong Ocean University, 524088 Zhanjiang, China
- Bio-Innovation Research Center, Tokushima University, 7793233 Tokushima, Japan
- Faculty of Bioscience and Bioindustry, Tokushima University, 7793233 Tokushima, Japan
*Corresponding author’s E-mail: dtklanh@vnua.edu.vn
Abstract
Background
Cryopreservation of bovine zygotes allows for a flexible schedule of genome editing via electroporation. However, vitrification-induced cell membrane damage may not only affect embryonic development but also genome mutation.
Objective
To investigate the effects of vitrification of zygotes before and after electroporation treatments on the development and genome mutation of bovine presumptive zygotes.
Materials and methods
In vitro-derived bovine zygotes were electroporated with the CRISPR/Cas9 system immediately (Vitrified-EP) or 2 h after incubation (Vitrified-2h-EP) following vitrification and warming, or electroporated before vitrification (EP-vitrified).
Results
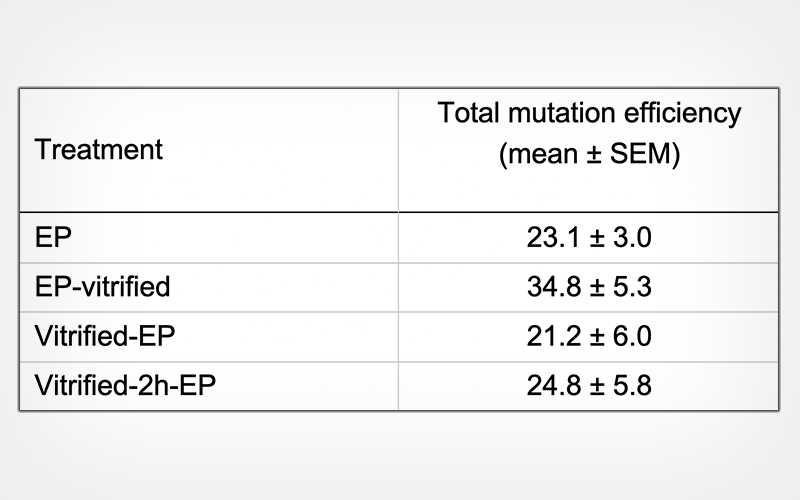
The development rates of vitrified-warmed zygotes were significantly lower (p < 0.05) than those of control zygotes that were not vitrified. Moreover, no differences were observed in the mutation rates and mutation efficiency of the blastocysts resulting from electroporated zygotes, irrespective of the timing of electroporation treatment.
Conclusion
Our results suggest that vitrification before and after electroporation treatments does not affect the genome editing of zygotes.
Keywords: bovine; CRISPR/Cas9; electroporation; MSTN; vitrification