CryoLetters Volume 43 - Issue 5
CryoLetters 43 (5), 255-263 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110112
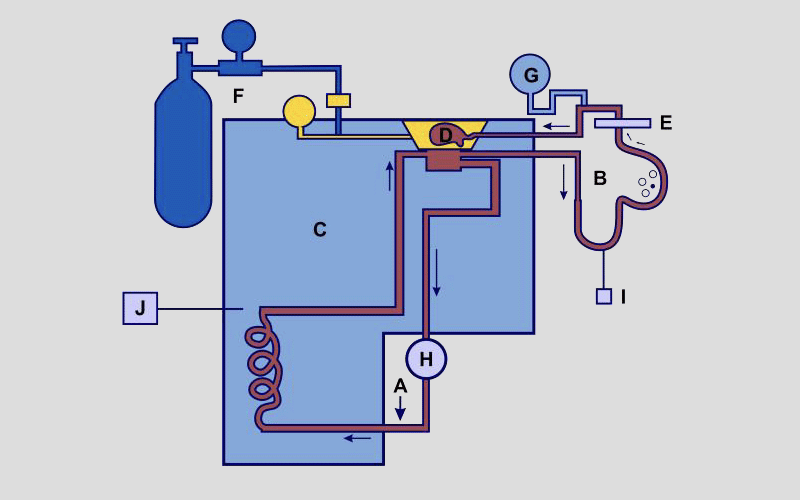
PERSPECTIVE: Hypothermic organ perfusion in the 2020s: mixing the benefits of low temperatures and dynamic flow outside the body
Daria Cherkashina1, Oleksandr Petrenko1 and Barry Fuller2*
- Institute for Problems of Cryobiology and Cryomedicine of NAS of Ukraine, Kharkiv, Ukraine
- Division of Surgery & Interventional Science, University College London Medical School, London, UK
*Corresponding author’s E-mail: b.fuller@ucl.ac.uk
Abstract
The cold chain supply of donor organs for transplantation has been an integral part of the delivery of transplant clinical services over the past five decades. Within the technologies used for this, hypothermic machine perfusion (HMP) was a concept, which was attractive to maintain organs under optimal conditions outside the body, and many early research studies on HMP were reported. However, it took the arrival of important new concepts to ensure that HMP was logistically feasible and valuable from an organ physiology perspective within the clinical pathways. This review provides details of the current status of HMP across the range of organs transplanted in the clinic, and discusses what new areas might benefit from applying HMP in coming years. In conclusion, HMP is now being used more frequently for clinical organ preservation in a variety of settings. As new therapies such as cell or gene therapy become more common, HMP will continue to play an important facilitator role for optimising organs in the donor pathway.
Keywords: dynamic cold perfusion; hypothermic machine perfusion; organ perfusion systems; organ preservation
CryoLetters 43 (5), 264-268 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110312
Trehalose in extenders for cryopreservation of Tambaqui (Colossoma macropomum) sperm
A.S. Varela Junior1, R.D. Jardim1, D.P. Streit Jr.2, T.F. Cardoso1, E.F. Silva1, T. Lucia, Jr.3, M.R.C. Figueiredo4 and C.D. Corcini3*
- RAC- Reprodução Animal Comparada, Instituto de Ciências Biológicas
- Aquam, Departamento de Zootecnia, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil
- Instituto de Oceanografia, Universidade Federal do Rio Grande, Rio Grande
- ReproPel, Faculdade de Veterinária, Universidade Federal de Pelotas, Pelotas
*Corresponding author’s E-mail: corcinicd@gmail.com
Abstract
Background
Sugars may act as either energy substrates or non-penetrating cryoprotectants.
Objective
Inclusion of non-penetrating trehalose was tested in extenders for the cryopreservation of Tambaqui (Colossoma macropomum) sperm.
Materials and methods
Sperm was extended 1/9 (v/v) in Beltsville Thawing Solution (BTS) with 10% DMSO (control) or 50, 100, 150 and 200 mM trehalose without 10% DMSO. Post-thawed sperm quality was evaluated, including fertilization and hatching rates, sperm motility, motility period and viability, integrity of sperm membrane and DNA, and mitochondrial functionality.
Results
Extenders with 100 ~ 150 mM trehalose achieved fertilization and hatching rates similar to those of the 10% DMSO-treated sperm samples. Trehalose at 100 and 150 mM provides better protection than 10% DMSO treatment for sperm motility, viability, DNA integrity and mitochondrial functionality. Fertilization and hatching rates were highly correlated (r = 0.95, P < 0.001).
Conclusion
The addition of 100 ~ 150 mM trehalose in extender can replace 10% DMSO for the cryopreservation of C. macropomum sperm.
Keywords: fish; membrane integrity; trehalose

CryoLetters 43 (5), 269-275 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110512
Pregnancy of cryopreserved ovine embryos at different developmental stages
Yanhua Guo1,#, Jiachen Bai 1,2,3,#, Zhenliang Zhang1, Yucheng Liu1, Shouliang Lu1, Changbin Liu1, Jianhong Ni1, Ping Zhou1, Xiangwei Fu1,3, Wendell Q. Sun2, Pengcheng Wan1* and Guoqing Shi1*
- State Key Laboratory of Sheep Genetic Improvement and Healthy Breeding, Institute of Animal Husbandry and Veterinary Sciences, Xinjiang Academy of Agricultural and Reclamation Sciences, Shihezi, Xinjiang, P.R. China
- Institute of Biothermal Science and Technology, School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai, P.R. China
- National Engineering Laboratory for Animal Breeding, Beijing Key Laboratory for Animal Genetic Improvement, College of Animal Science and Technology, China Agricultural University, Beijing, P.R. China
*Corresponding authors’ E-mail: pengcheng.wan@gmail.com and nkkxyxms@163.com
#Both authors contributed equally to this paper.
Abstract
Background
Developmental stage and cryopreservation method have significant impact on the pregnancy rate after transfer of embryos produced in vivo.
Objective
To determine the pregnancy outcomes from ovine embryos cryopreserved at different developmental stages.
Materials and methods
Embryos at different developmental stages were obtained from donor ewes through simultaneous estrus treatment and laparoscopic artificial insemination. Embryos, either cryopreserved via vitrification or slow freezing method, were implanted into recipient ewes. The pregnancy rate was determined 35 days after transfer.
Results
The pregnancy rate of developing embryos increases after transfer from the morula stage, early blastocyst to expanded blastocyst stages (64.9%, 73.9% and 81.3%, respectively). However, cryopreservation significantly decreases the pregnancy rate of embryos at all three developmental stages, and there is no significant difference among developmental stages (43.9%, 43.7%, 52.9%, respectively). There is also no significant difference in the pregnancy rate between slowly-frozen embryos and vitrified embryos.
Conclusion
The pregnancy outcomes of embryo transfer is better at the expanded blastocyst stage than at earlier stages. However, no difference is observed in the pregnancy rate of embryos at different developmental stage after cryopreservation, either by slow freezing and vitrification. Cryopreservation methods for ovine embryos, both slow freezing and vitrification, need further improvement.
Keywords: cryopreserved methods; embryo; embryonic stages; ovine

CryoLetters 43 (5), 276-282 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110712
Cryoprotection of humanin-like peptides in seminal plasma for ejaculated spermatozoa of crossbred bulls
Megha Pande1*, S.K. Ghosh2, S. Tyagi1, R. Katiyar2, N. Srivastava2, M. Karikalan3, S. Kumar1, K. Krishnappa4, A.S. Sirohi1, Sarika1 and A. Mitra1
- Division of Cattle Physiology and Reproduction, ICAR-Central Institute for Research on Cattle, Meerut, Uttar Pradesh, India
- Division of Animal Reproduction, ICAR-Central Institute for Research on Cattle, Meerut, Uttar Pradesh, India
- Division of Veterinary Pathology, ICAR-Central Institute for Research on Cattle, Meerut, Uttar Pradesh, India
- Division of Medicine, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India
*Corresponding author’s E-mail: Megha.Pande@icar.gov.in
Abstract
Background
Cryopreservation process negatively affects spermatozoa functions. Humanin, a small polypeptide encoded in the mitochondrial genome, is well known for its role in cell survival.
Objective
To quantify the endogenous levels of humanin in seminal plasma of crossbred Frieswal bulls and to study its role in cryoprotection. The presence of humanin in bull spermatozoa was also investigated.
Materials and methods
A total of 40 semen samples were separated into two groups based on the initial progressive motility (IPM): Good (IPM≥70%) and Poor (IPM≤50%) groups; and/or based on the post-thaw motility (PTM): Freezable (PTM≥50%) and Non-freezable (PTM<50%) groups. Humanin concentration in seminal plasma (SP-HN) was quantified using ELISA.
Results
SP-HN concentration ranged from undetectable to 67.6 pg/mL with a median level of 35.2 pg/mL. SP-HN level was significantly higher in the good quality semen group than in the poor quality semen group (p<0.001), and also significantly higher in the freezable group than in the non-freezable group (p<0.001). SP-HN level was positively correlated with initial progressive motility, post-thaw semen motility, viability, acrosome intactness and plasma membrane integrity, but negatively correlated the level of reactive oxygen species and malondialdehyde content. Immunochemical localization showed the presence of humanin in the proximal region of the middle piece of spermatozoa.
Conclusion
Endogenous humanin level had significant correlation with semen quality and might protect sperm cells against freeze-induced oxidative stress.
Keywords: crossbred bulls; Frieswal; humanin; oxidative stress; semen cryopreservation

CryoLetters 43 (5), 283-288 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110212
Effect of betaine and raffinose in cryopreservation medium on fertility in Kadaknath chicken
Pranay Balusa1, Swathi Bommu1 and Shanmugam Murugesan2*
- Department of Physiology, College of Veterinary Science, PVNRTVU, Hyderabad, India
- ICAR-Directorate of Poultry Science, Rajendranagar, Hyderabad, India
*Corresponding author’s E-mail: shanmugam.murugesan@icar.gov.in
Abstract
Background
Kadaknath is an important indigenous chicken with black pigmentation and cryopreserved semen reputably had low fertility.
Objective
The aim of this study was to evaluate the effects of betaine and raffinose in semen extenders on post thaw semen parameters and fertility.
Materials and methods
Semen was cryopreserved in 4% dimethyl sulfoxide (DMSO) with betaine supplemented at 0.1, 0.2 and 0.4 M or raffinose supplemented at 1, 5 and 10 mM. Post thaw semen parameters and fertility were evaluated.
Results
Betaine at higher concentrations significantly (p < 0.05) inhibited the post thaw sperm motility, live sperm and MTT dye reduction and a declining trend in the fertility with increasing betaine. Inclusion of raffinose had no effect on the post thaw in vitro semen parameters, however, the fertility was significantly (p < 0.05) higher in the 10 mM raffinose supplemented group.
Conclusion
Betaine has negative effect on post thaw semen parameters and raffinose at 10 mM concentration improves the fertility from cryopreserved semen.
Keywords: betaine; chicken; cryopreservation; fertility; raffinose; Kadaknath breed

CryoLetters 43 (5), 289-294 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110412
Effect of ovarian storage temperature and time on post thaw viability and maturation rate of vitrified immature oocytes in sheep
Khursheed Ahmad Sofi1* and Beenish Qureshi2
- Assistant Professor, Division of Veterinary Clinical Complex, F.V.Sc and A.H, Shuhama, SKUAST-Kashmir, Srinagar, India
- JRF, Division of Veterinary Clinical Complex, F.V.Sc and A.H, Shuhama, SKUAST-Kashmir, Srinagar, India
*Corresponding author’s E-mail: drsofi.vet54321@gmail.com
Abstract
Background
Vitrification of oocytes as a method of cryopreservation is quite successful, although it is still being standardized because of structural and molecular sensitivity of oocytes to the cooling and freezing process.
Objective
To investigate the effect of ovarian storage temperature and time on post thaw viability and maturation rate of vitrified immature oocytes in sheep.
Materials and methods
The work consisted of oocyte collection from ovaries of abattoir sheep stored at various temperature (0°C, 4°C and 25°C) and time (0 h, 6 h, 12 h and 24 h) combinations and post thaw viability and in vitro maturation rate evaluation. Vitrification was done in 30% vitrification solution, using ethylene glycol and DMSO, with post vitrification evaluation after 1 week’s storage.
Results
Significantly higher post thaw viability was observed after storage at 0°C for 6 h (95.3%) followed by 12 h (85%), with lowest value at 24 h (66.7%). However at 4°C and 25°C, values were non-significantly higher after 6 h (96.5 and 100% respectively) followed by 12 h (93 and 100%), with significantly lower values after 24 h (85.7 and 90.7%). At storage temperatures of 25°C and 4°C, a significantly higher percentage of mature oocytes was observed after 6 h (40 and 39.1%), 12 h (37.3 and 38.1%) and 24 h (34.6 and 36.4%) storage times compared to that at 0°C (20.3% at 6 h, 14.2% at 12 h and only 13.8% at 24 h). However, at all storage temperatures, there was a tendency for the level of mature oocytes to decrease with storage time, and the levels were significantly lower than the control.
Conclusion
Acceptable post thaw viability and in vitro maturation rates for oocytes is maintained up to 24 h in ovaries stored at 4°C and 25°C compared to at 0°C, and these conditions may be used for the storage of ovaries meant for oocyte preservation.
Keywords: maturation; temperature; time; viability; vitrification

CryoLetters 43 (5), 295-302 (2022)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr22510110612
Artificial seed production and cryopreservation by encapsulation dehydration for medicinal herb of Himalayan region, Swertia chirayita
Rolika Gupta and Hemant Sood*
- Jaypee University of Information Technology, Department of Biotechnology and Bioinformatics, Waknaghat, Solan, Himachal Pradesh. 173234, India
*Corresponding author’s E-mail: hemant.sood@juit.ac.in; Orcid ID: 0000-0003-0001-4317
Abstract
Background
Cryopreservation of germplasm in liquid nitrogen is an ideal technique for the longer term storage of plant genetic material, including medicinal species.
Objective
To develop a somatic embryo production system for the medicinal species Swertia chirayita and to evaluate their potential for storage in liquid nitrogen (-196°C).
Materials and methods
An efficient protocol of somatic embryogenesis was developed for the first time using leaves of in-vitro grown shoots of S. chirayita. Somatic embryos were then encapsulated in 3% sodium alginate, 0.85 M sucrose and 100 mM calcium chloride for synthetic seed production and subjected to cryopreservation. Marker medicinal compounds were determined by RP-HPLC analysis.
Results
A medium containing 1 mg/L 2,4-D+ 0.5 mg/L BAP+ 0.5 mg/L TDZ was found to stimulate the highest callus induction. Somatic embryos were recovered after 5 weeks, when cultured on the same media. Synthetic seeds were dehydrated and immersed in liquid nitrogen for 1 h. Cryopreserved synthetic seeds were successfully revived and germinated on MS media supplemented with 1 mg/L IBA+ 2 mg/L KN + 3 mg/L GA3 in which 93.3% somatic embryos differentiated into shoots. One month old in-vitro grown shoots from cryopreserved somatic embryos had similar marker medicinal compounds, such as amarogentin (4.72 ± 0.11 μg/mg) and mangiferin (14.54 ± 0.05 μg/mg), as control material.
Conclusion
This protocol offers vast scope for multiplying material of an endangered medicinal herb and subsequent cryopreservation.
Keywords: cryopreservation; MS media; somatic embryo; Swertia chirayita; synthetic seed







